
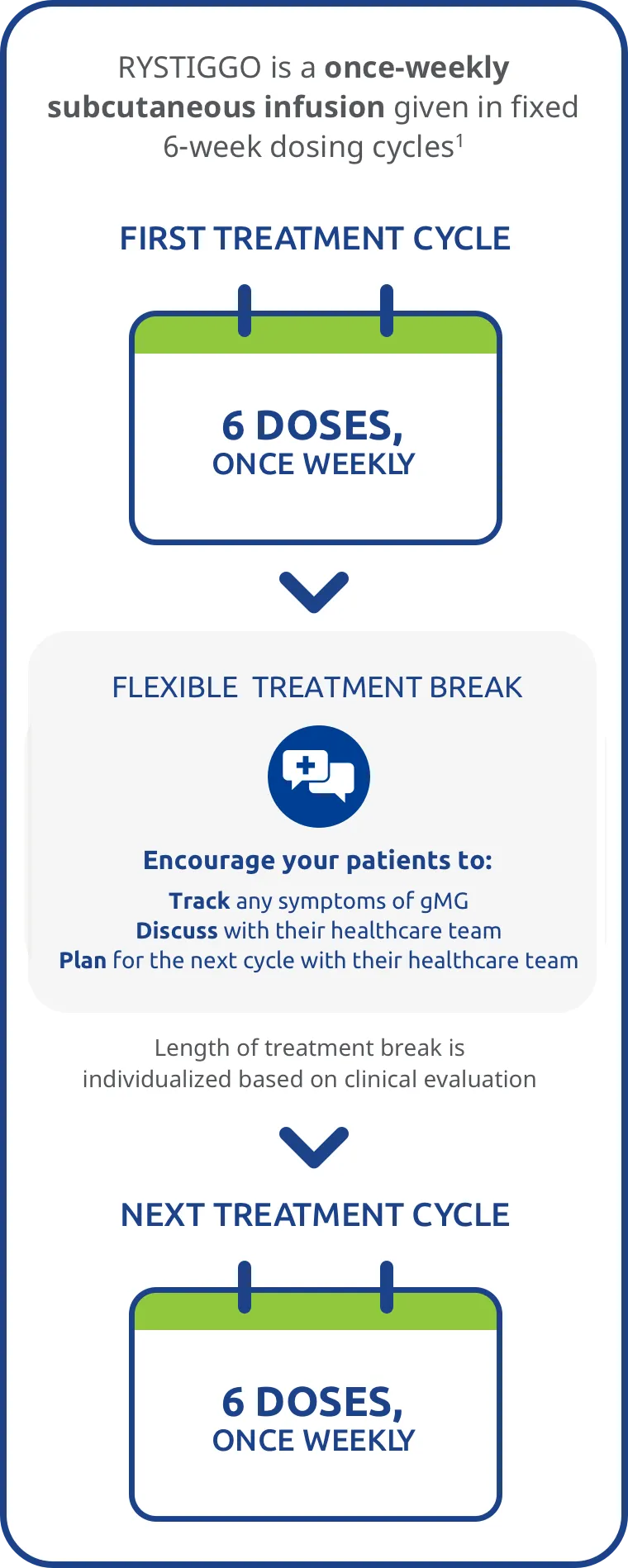
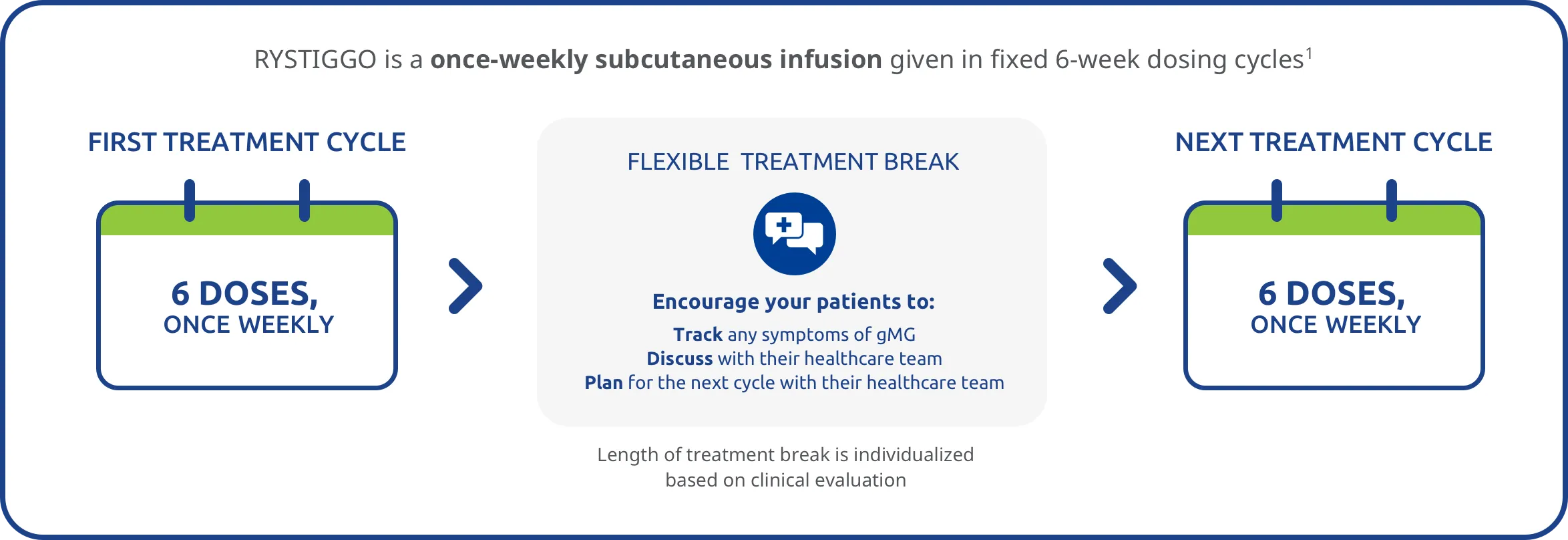
RYSTIGGO provides cyclical dosing with a fixed 6-week dosing cycle followed by a flexible, individualized break in treatment1*†
See when RYSTIGGO reaches maximum efficacy during the treatment cycle
In RYSTIGGO clinical studies1:
- 8 weeks of observation followed the 6-week treatment period
- The safety of initiating subsequent cycles sooner than 9 weeks (63 days) from the start of the previous treatment cycle has not been established
- 4 treatment cycles were initiated per year, on average (range: 1-7 cycles)
Encourage your patients to keep track of gMG symptoms between their treatment cycles
RYSTIGGO dosage is based on body weight1
420 mg/
3 mL
(140 mg/mL)
560 mg/
4 mL
(140 mg/mL)
840 mg/
6 mL
(140 mg/mL)
- RYSTIGGO is administered as a subcutaneous infusion once weekly for 6 weeks1
- If a scheduled infusion is missed, RYSTIGGO may be administered up to 4 days after the scheduled time point1
- Thereafter, resume the original dosing schedule until the treatment cycle is completed1
RYSTIGGO is a once-weekly subcutaneous infusion administered in approximately 15 minutes once preparation is complete1

 Administration:
Administration:
- Time of administration may vary by patient. Duration of infusion may be longer based on flow rate and patient weight1
- RYSTIGGO is intended to be infused in the lower right or lower left part of the abdomen below the navel1
- Do not infuse where the skin is tender, bruised, red, or hard. Avoid infusing into tattoos, scars, or stretch marks1
- Rotate infusion sites for subsequent administrations1
 Observation:
Observation:
- Monitor patients during treatment with RYSTIGGO and for 15 minutes after completion for clinical signs and symptoms of hypersensitivity reactions. If a reaction occurs, discontinue administration of RYSTIGGO and institute appropriate measures if needed1
- In clinical trials, hypersensitivity reactions occurred within 1 day to 2 weeks of administration. One patient discontinued RYSTIGGO due to a hypersensitivity reaction. Local reactions at the administration site occurred within 1 to 3 days after the most recent RYSTIGGO infusion1

Get your quick reference guide on preparing and performing infusion with RYSTIGGO
RYSTIGGO infusions can be administered in different settings
RYSTIGGO is for subcutaneous administration only using an infusion pump. Refer to the infusion pump manufacturer’s instructions for full preparation and administration information. It is recommended to use pumps where administered volume can be preset, as each vial contains excess volume for priming of the infusion line.1
Physician office infusion suite
These physician offices have capabilities to both prescribe and infuse RYSTIGGO.
Register your practice with the RYSTIGGO Infusion Center Finder
Independent infusion center
These centers are independent from physician offices and hospitals. Infusion nurses staff these centers; physicians may or may not be on-site.
Find an infusion center for your gMG patients
Home infusion
Some patients may be eligible for RYSTIGGO to be administered at home by a trained nurse. Eligibility is based on a patient’s insurance plan. Not all patients are eligible.
Hospital outpatient department
These facilities exist within hospitals for patients who do not need to be admitted to receive infusions.
Administration considerations with RYSTIGGO1
Infections
- Delay RYSTIGGO administration in patients with an active infection until the infection is resolved
Immunization
- Because RYSTIGGO causes a reduction in IgG levels, vaccination with live-attenuated or live vaccines is not recommended during treatment with RYSTIGGO
Hypersensitivity Reactions
- If a hypersensitivity reaction occurs, the healthcare professional should institute appropriate measures if needed or the patient should seek medical attention
Effect of RYSTIGGO on other drugs
- Concomitant use of RYSTIGGO with medications that bind to the human neonatal Fc receptor (FcRn) (eg, immunoglobulin products, monoclonal antibodies, or antibody derivatives containing the human Fc domain of the IgG subclass) may lower systemic exposures and reduce effectiveness of such medications
Watch the administration video for step-by-step instructions on how to properly administer RYSTIGGO
RYSTIGGO is intended for subcutaneous administration using an infusion pump at a constant flow rate of up to 20 mL/hr.¹
If a scheduled infusion is missed, RYSTIGGO may be administered up to 4 days after the scheduled time point. Thereafter, resume the original dosing schedule until the treatment cycle is completed.1
Fc=fragment crystallizable; gMG=generalized myasthenia gravis; IgG=immunoglobulin G; MG-ADL=Myasthenia Gravis Activities of Daily Living.
Reference:
- RYSTIGGO [Prescribing Information]. Smyrna, GA: UCB, Inc.




