
SAFETY
MycarinG CLINICAL TRIAL
RYSTIGGO safety was demonstrated in a Phase 3 clinical study1
Adverse reactions occurring in ≥5% of adults treated with RYSTIGGO and more frequently than in adults treated with placebo1
The most common adverse reactions (≥10%) in adults treated with RYSTIGGO were headache, infections, diarrhea, pyrexia, hypersensitivity reactions, and nausea.1
RYSTIGGO warnings and precautions
Infections1
RYSTIGGO may increase the risk of infection.
- Delay RYSTIGGO administration in patients with an active infection until the infection is resolved
- During treatment with RYSTIGGO, monitor for clinical signs and symptoms of infection
- If serious infection occurs, administer appropriate treatment and consider withholding RYSTIGGO until the infection has resolved
In clinical studies, common infections (at least 5% frequency) were upper respiratory tract infections (17%), COVID-19 (14%), urinary tract infections (9%), and herpes simplex (6%). Serious infections were reported in 4% of patients treated with RYSTIGGO. Three fatal cases of pneumonia were identified caused by COVID-19 infection in two patients and an unknown pathogen in one patient. Six cases of infections led to discontinuation of RYSTIGGO.
Immunization
Immunization with vaccines during RYSTIGGO treatment has not been studied. The safety of immunization with live or live-attenuated vaccines and the response to immunization with any vaccine are unknown. Because RYSTIGGO causes a reduction in IgG levels, vaccination with live-attenuated or live vaccines is not recommended during treatment with RYSTIGGO. Evaluate the need to administer age-appropriate vaccines according to immunization guidelines before initiation of a new treatment cycle with RYSTIGGO.
Aseptic Meningitis1
Serious adverse reactions of aseptic meningitis (also called drug-induced aseptic meningitis) have been reported in patients treated with RYSTIGGO.
- If symptoms consistent with aseptic meningitis develop, diagnostic workup and treatment should be initiated according to the standard of care
Hypersensitivity Reactions1
Hypersensitivity reactions, including angioedema and rash, were observed in patients treated with RYSTIGGO.
- Management of hypersensitivity reactions depends on the type and severity of the reaction
- Monitor patients during treatment with RYSTIGGO and for 15 minutes after the administration is complete for clinical signs and symptoms of hypersensitivity reactions
- If a hypersensitivity reaction occurs, the healthcare professional should institute appropriate measures if needed or the patient should seek medical attention
EXTENSION STUDIES
RYSTIGGO safety was further evaluated in the extension studies, with primary endpoints assessing the proportion of patients experiencing TEAEs3,4
TEAEs reported in >10% of adults in at least one of the RYSTIGGO treatment groups5
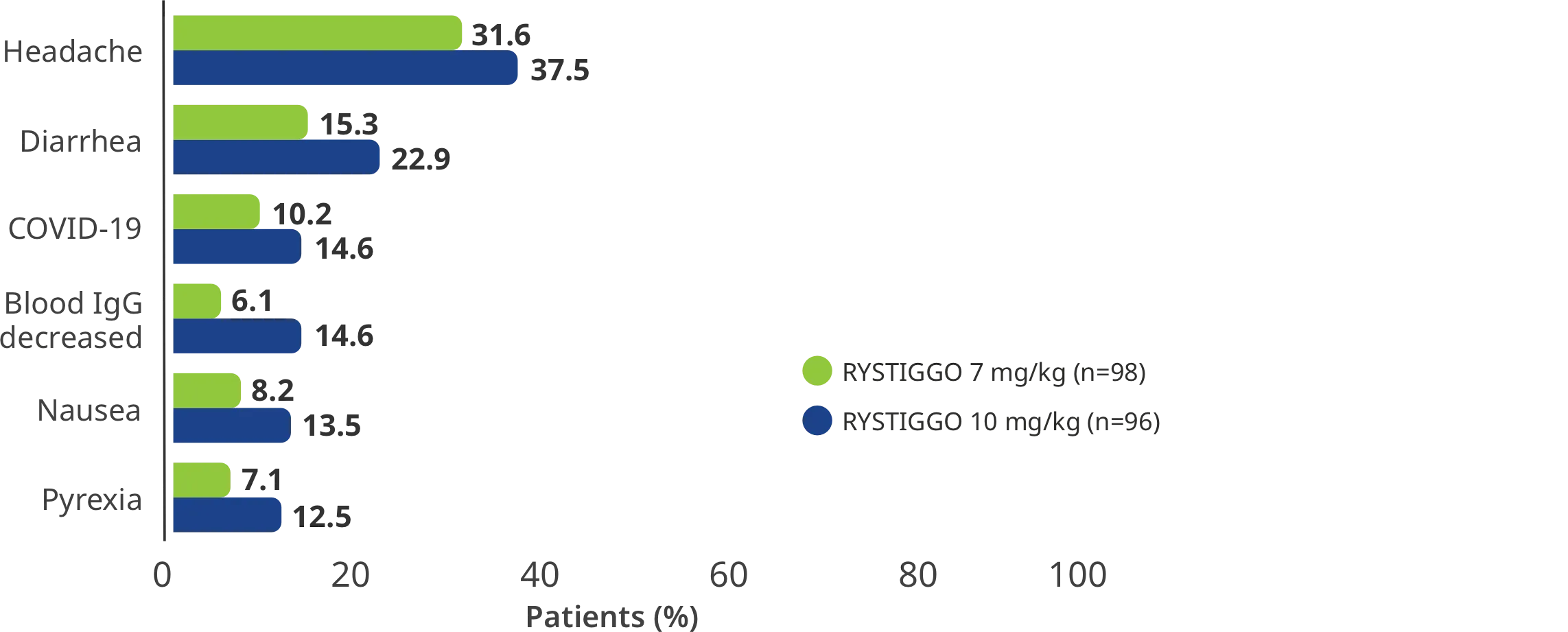
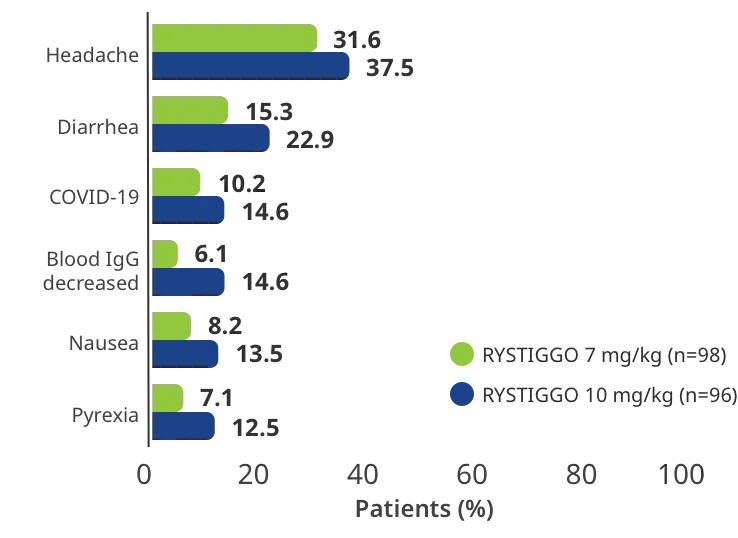
Interim analysis as of July 8, 2022.5
COVID-19=coronavirus disease 2019; IgG=immunoglobulin G; TEAEs=treatment-emergent adverse events.
References:
- RYSTIGGO [Prescribing Information]. Smyrna, GA: UCB, Inc.
- Bril V, Drużdż A, Grosskreutz J, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22(5):383-394. doi:10.1016/S1474-4422(23)00077-7
- A study to investigate the long-term safety, tolerability, and efficacy of rozanolixizumab in adult patients with generalized myasthenia gravis. ClinicalTrials.gov identifier: NCT04124965. Updated September 5, 2023. Accessed February 27, 2025. https://clinicaltrials.gov/study/NCT04124965
- A study to evaluate rozanolixizumab in study participants with generalized myasthenia gravis. ClinicalTrials.gov identifier: NCT04650854. Updated February 26, 2024. Accessed February 27, 2025. https://clinicaltrials.gov/study/NCT04650854
- Data on file. UCB, Inc., Smyrna, GA.



