
MECHANISM
OF ACTION
RYSTIGGO is a humanized monoclonal
antibody designed to block FcRn
in adults with anti-AChR Ab+ or
anti-MuSK Ab+ gMG1,2*
The precise mechanism through which RYSTIGGO exerts therapeutic effects in gMG is unknown.
RYSTIGGO is a humanized monoclonal antibody designed to block FcRn in adults with anti-AChR Ab+ or anti-MuSK Ab+ gMG1,2*
The precise mechanism through which RYSTIGGO exerts therapeutic effects in gMG is unknown.
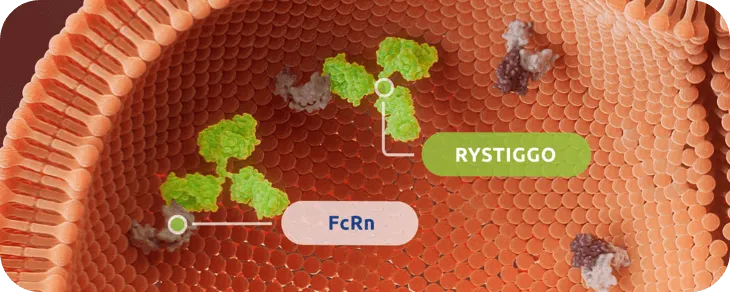
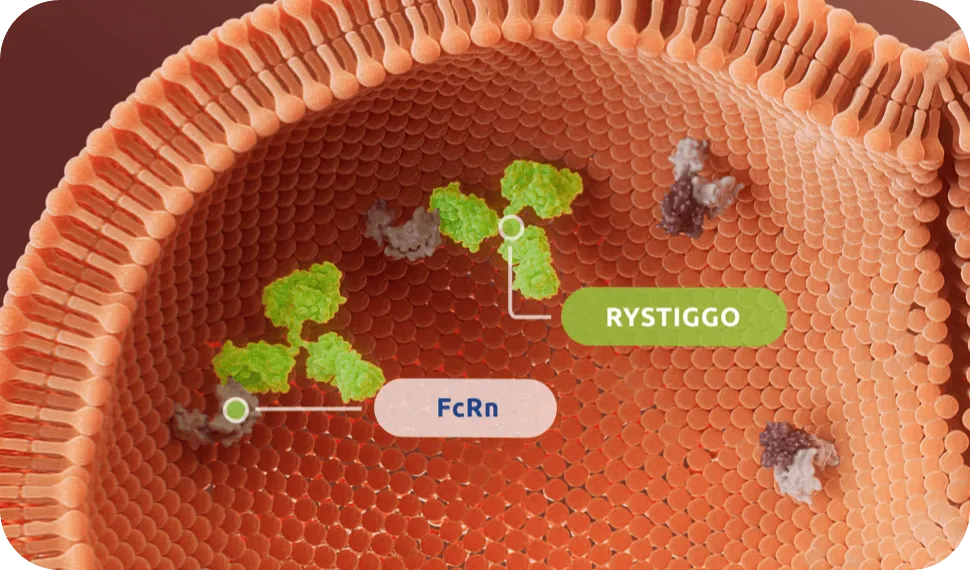
RYSTIGGO is engineered to block FcRn1
RYSTIGGO specifically targets and blocks IgG antibodies from binding to FcRn.1,2
FcRn is a receptor expressed on the endothelial cells that mediates a natural salvage and recycling mechanism. IgG antibodies bound to FcRn are returned to circulation and escape lysosomal degradation.3
High-Affinity Binding
RYSTIGGO binds to FcRn with high affinity, reducing IgG recycling and leading to degradation.1,4†
Clinical data with RYSTIGGO have not identified any clinically relevant impact on levels of albumin, which binds at a different site on FcRn.4,5
Learn more about the pharmacodynamic effects of RYSTIGGO in the secondary endpoints efficacy section
Discover how RYSTIGGO works
Learn more about how blocking FcRn removes AChR and MuSK autoantibodies.1
Press play to watch the full video or select a chapter that interests you:
RYSTIGGO Indication
gMG MOD
AChR and MuSK Autoantibodies
Antibody Recycling in gMG
RYSTIGGO MOA
Important Safety Information
RYSTIGGO® (rozanolixizumab-noli) is indicated for the treatment of generalized myasthenia gravis, also known as gMG, in adult patients who are anti-acetylcholine receptor, also called AChR, or anti–muscle-specific tyrosine kinase, or MuSK, antibody positive.1
Please see Important Safety Information for RYSTIGGO at the end of this video.
In gMG, pathogenic immunoglobulin G, or IgG, autoantibodies attack key proteins on the postsynaptic membrane involved in signal transmission at the neuromuscular junction, or NMJ.2,3 This impairs signal transduction and muscle contraction, which leads to the debilitating muscle weakness and fatigue experienced by patients with gMG.2,3
AChR and MuSK are among the key proteins on the postsynaptic membrane affected in gMG.3
Pathogenic autoantibodies to either AChR or MuSK are found in approximately 92%-95% of people living with gMG.4,5
AChR autoantibodies result in pathology via any of the following 3 mechanisms:
1. Preventing acetylcholine from binding to its receptor and thus inhibiting neurotransmission3
2. Reducing receptor density because of cross-linking and subsequent AChR endocytosis, leading to lower signaling capacity3
3. Activating the complement system leads to the formation of a membrane attack complex, or MAC, essentially puncturing the postsynaptic membrane3,4
MuSK autoantibodies also exert a pathogenic effect in gMG.3,5 Normally, when agrin-LRP4 complex activates MuSK, AChRs cluster, leading to an increase in the efficiency of signal transmission following acetylcholine binding.3,5-7 In gMG, even though MuSK autoantibodies do not activate the complement pathway, they contribute to a progressive loss of acetylcholine receptors and subsequent synaptic failure—and may be associated with more severe manifestations of the disease.3,5
Pathogenic autoantibodies causing gMG can remain elevated in serum by utilizing an escape mechanism to avoid lysosomal degradation within endothelial cells.2,3,8
Inside the endothelial cell, AChR and MuSK autoantibodies bind to neonatal Fc receptors, or FcRns, escape lysosomal degradation, and are recycled, returning to the circulation.2,3,8 Thus, FcRns help to extend the half-life of AChR and MuSK autoantibodies and perpetuate symptoms of gMG.2,3,8,9
RYSTIGGO is an FcRn blocker and a targeted therapy for gMG.1 It is the first monoclonal antibody, or mAb, specifically designed to bind to FcRn with high affinity in adult patients with gMG who are anti-AChR or anti-MuSK antibody positive.1,8
High-affinity binding allows RYSTIGGO to compete with other IgGs—including AChR and MuSK pathogenic autoantibodies—for the site on FcRn.8 IgGs bound to FcRn are protected from lysosomal degradation.2 Unbound autoantibodies are vulnerable to lysosomal degradation.2 Trial data with RYSTIGGO have not identified any clinically relevant impact on levels of albumin, which binds at a different site on FcRn.9
IgG antibodies, including AChR and MuSK pathogenic autoantibodies, that are left unbound to FcRn are then destroyed in the lysosome.1-3,8
Lysosomal destruction of AChR and MuSK autoantibodies results in their clearance from circulation.1-3,8 That means there are fewer AChR and MuSK autoantibodies to interfere with the neuromuscular signaling that may lead to the debilitating muscle weakness that is a hallmark of gMG.1-3,8,9
Reducing the levels of pathogenic autoantibodies in adult patients with either anti-AChR or anti-MuSK antibody-positive gMG helps restore signaling at the neuromuscular junction.1-3,8,9 RYSTIGGO offers a targeted treatment option for a broad population of adults with gMG.1,4,5,9*
Please listen to the following Important Safety Information for RYSTIGGO.
INDICATION
RYSTIGGO (rozanolixizumab-noli) is indicated for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) or anti-muscle-specific tyrosine kinase (MuSK) antibody positive.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Infections: RYSTIGGO may increase the risk of infection. Delay RYSTIGGO administration in patients with an active infection until the infection is resolved. During treatment with RYSTIGGO, monitor for clinical signs and symptoms of infection. If serious infection occurs, administer appropriate treatment and consider withholding RYSTIGGO until the infection has resolved.
Immunization
Immunization with vaccines during RYSTIGGO treatment has not been studied. The safety of immunization with live or live-attenuated vaccines and the response to immunization with any vaccine are unknown. Because RYSTIGGO causes a reduction in IgG levels, vaccination with live-attenuated or live vaccines is not recommended during treatment with RYSTIGGO. Evaluate the need to administer age-appropriate vaccines according to immunization guidelines before initiation of a new treatment cycle with RYSTIGGO.
Aseptic Meningitis: Serious adverse reactions of aseptic meningitis (also called drug-induced aseptic meningitis) have been reported in patients treated with RYSTIGGO. If symptoms consistent with aseptic meningitis develop, diagnostic workup and treatment should be initiated according to the standard of care.
Hypersensitivity Reactions: Hypersensitivity reactions, including angioedema and rash, were observed in patients treated with RYSTIGGO. Management of hypersensitivity reactions depends on the type and severity of the reaction. Monitor patients during treatment with RYSTIGGO and for 15 minutes after for clinical signs and symptoms of hypersensitivity reactions. If a reaction occurs, institute appropriate measures if needed.
ADVERSE REACTIONS
In a placebo-controlled study, the most common adverse reactions (reported in at least 10% of RYSTIGGO-treated patients) were headache, infections, diarrhea, pyrexia, hypersensitivity reactions, and nausea. Serious infections were reported in 4% of patients treated with RYSTIGGO. Three fatal cases of pneumonia were identified, caused by COVID-19 infection in two patients and an unknown pathogen in one patient. Six cases of infections led to discontinuation of RYSTIGGO.
Please see the full Prescribing Information at www.RYSTIGGOhcp.com
†Based on in vitro data.4
Ab+=antibody positive; AChR=acetylcholine receptor; FcRn=neonatal Fc receptor; gMG=generalized myasthenia gravis; IgG=immunoglobulin G; MuSK=muscle-specific tyrosine kinase.
References:
- RYSTIGGO [Prescribing Information]. Smyrna, GA: UCB, Inc.
- Lledo-Garcia R, Dixon K, Shock A, et al. Pharmacokinetic-pharmacodynamic modelling of the anti-FcRn monoclonal antibody rozanolixizumab: translation from preclinical stages to the clinic. CPT Pharmacometrics Syst Pharmacol. 2022;11(1):116-128. doi:10.1002/psp4.12739
- Gable KL, Guptill JT. Antagonism of the neonatal Fc receptor as an emerging treatment for myasthenia gravis. Front Immunol. 2020;10(3052):1-9. doi:10.3389/fimmu.2019.03052
- Smith B, Kiessling A, Lledo-Garcia R, et al. Generation and characterization of a high affinity anti-human FcRn antibody, rozanolixizumab, and the effects of different molecular formats on the reduction of plasma IgG concentration. MAbs. 2018;10(7):1111-1130. doi:10.1080/19420862.2018.1505464
- Bril V, Drużdż A, Grosskreutz J, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22(5):383-394. doi:10.1016/S1474-4422(23)00077-7



