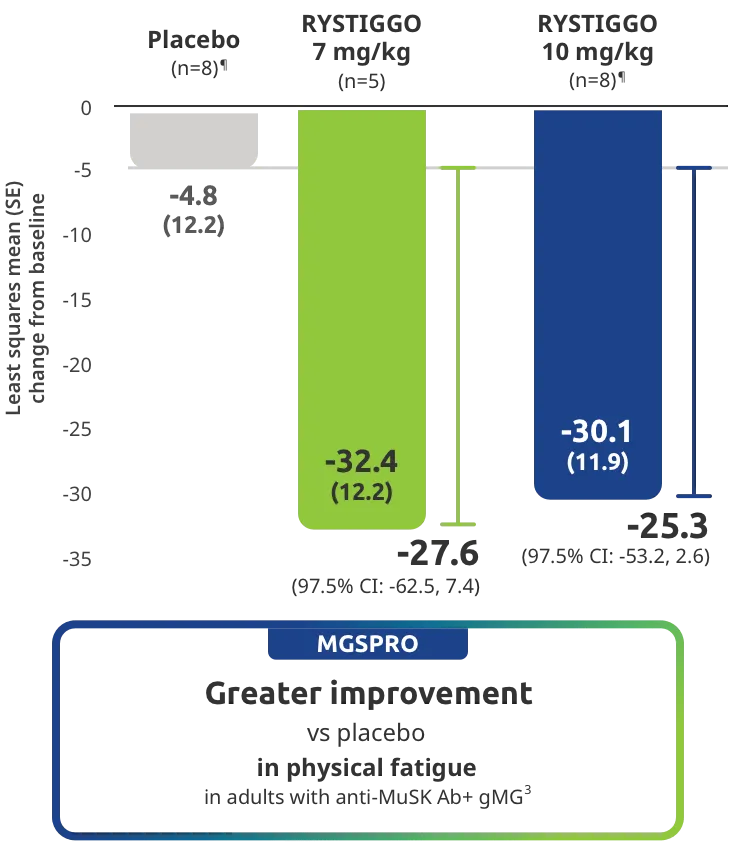
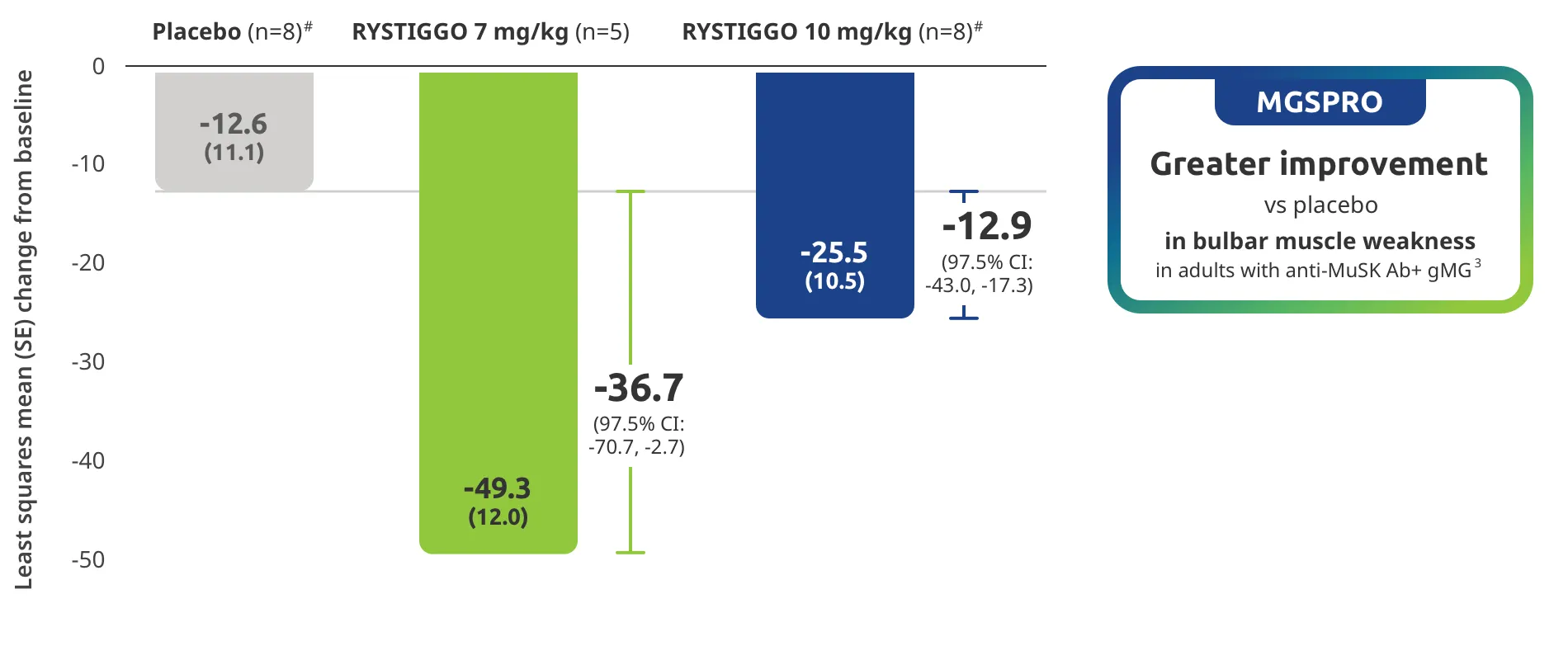
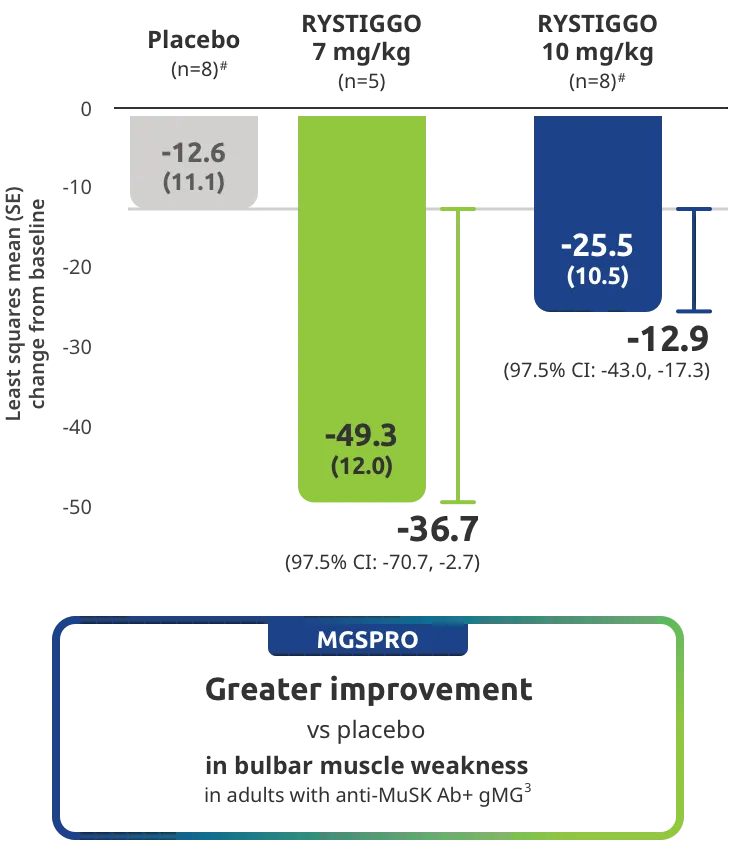
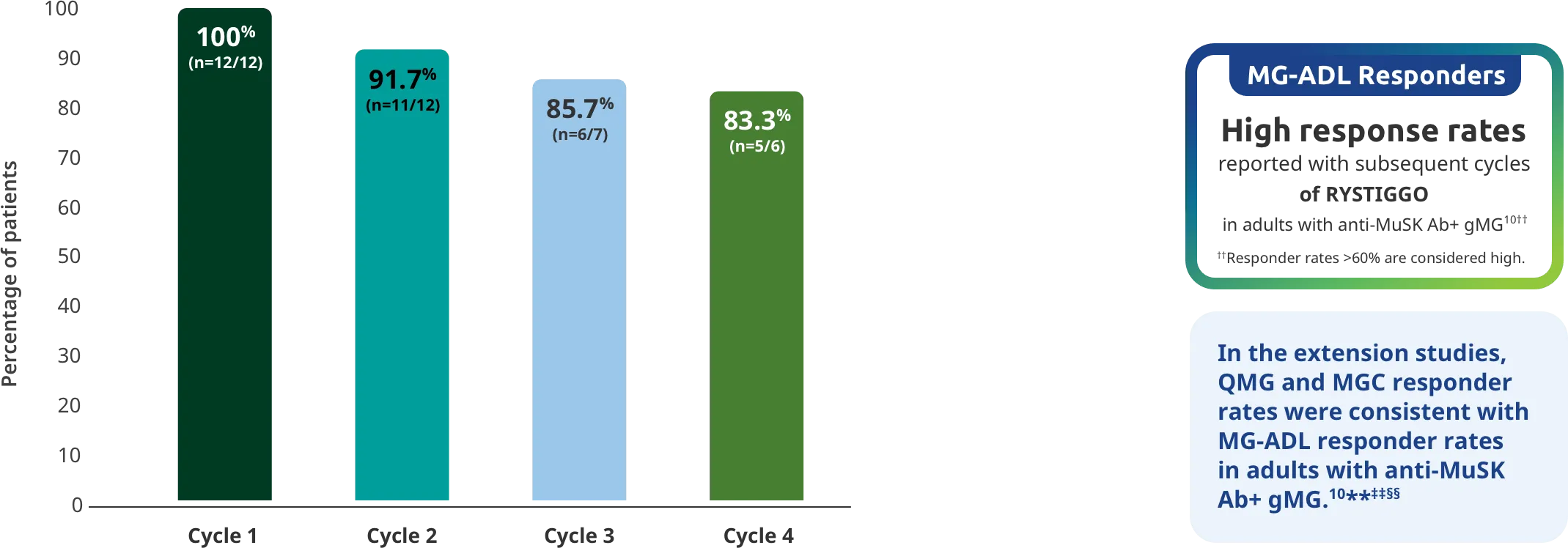
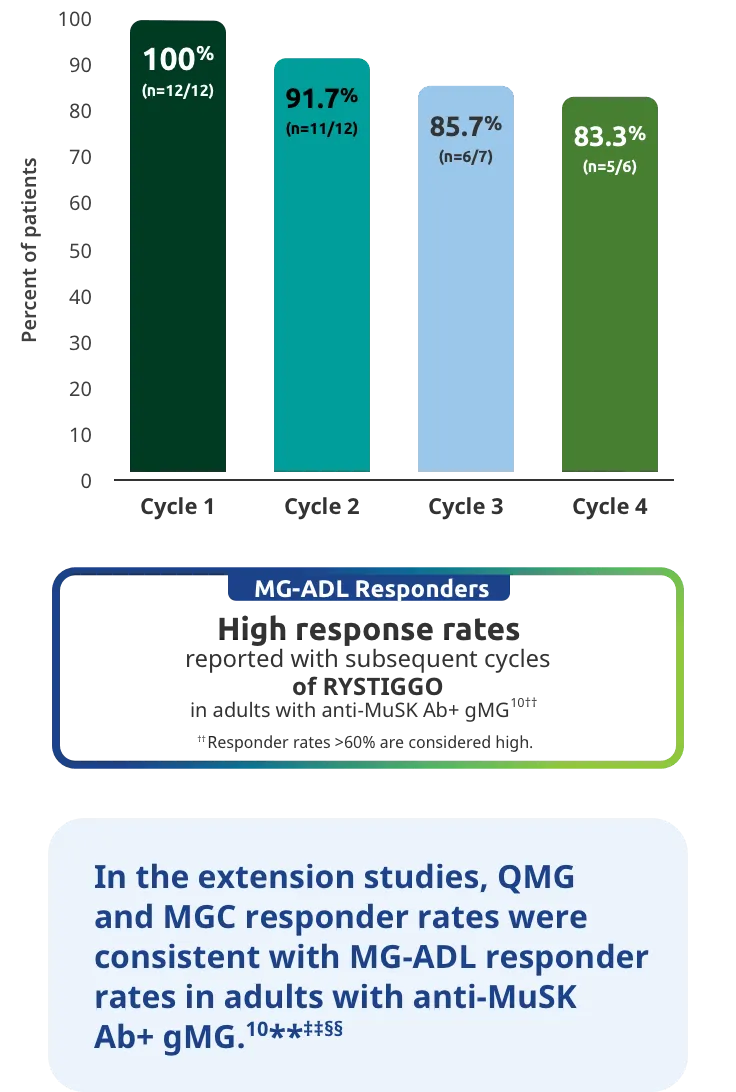
PRIMARY ENDPOINT: MG-ADL
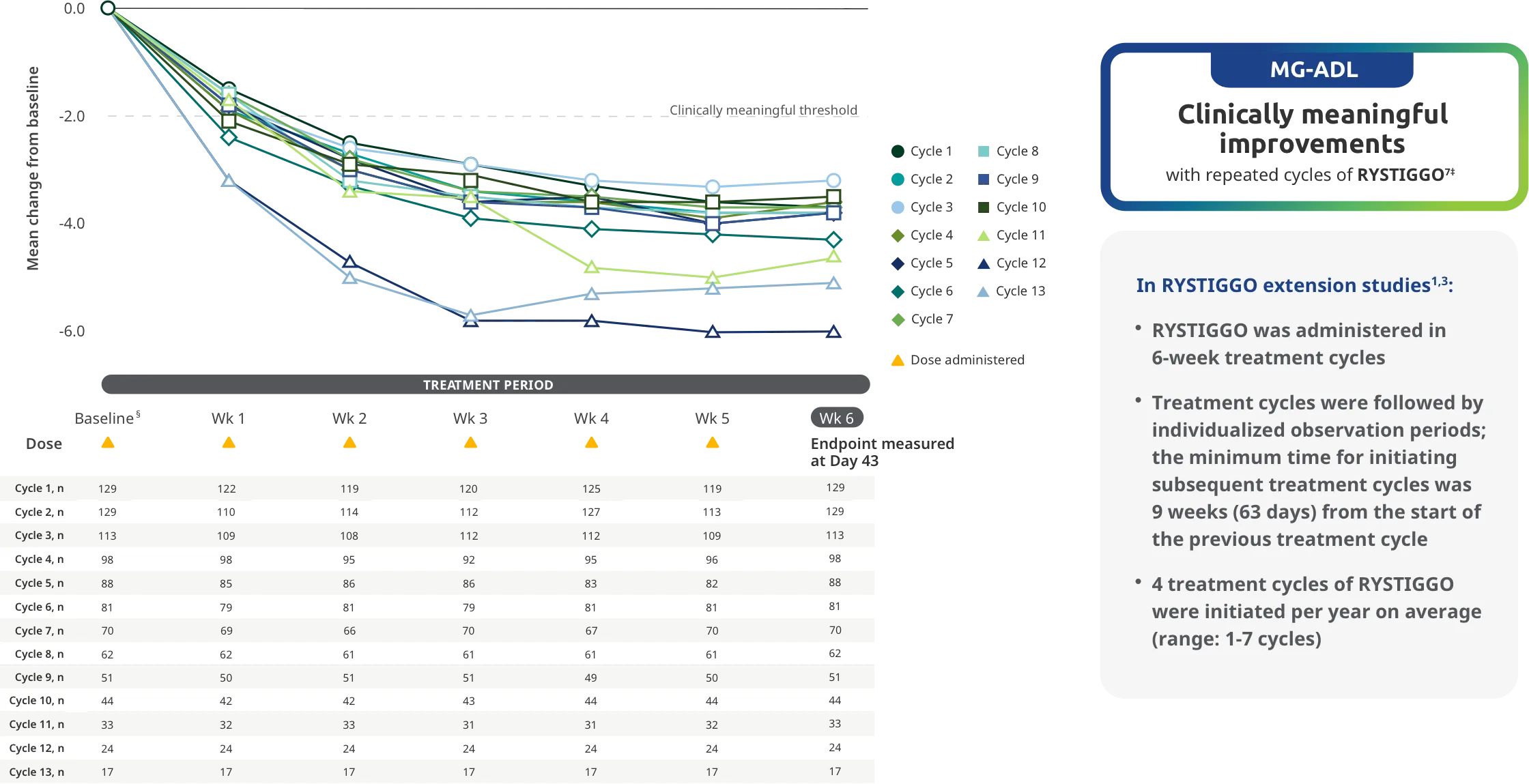
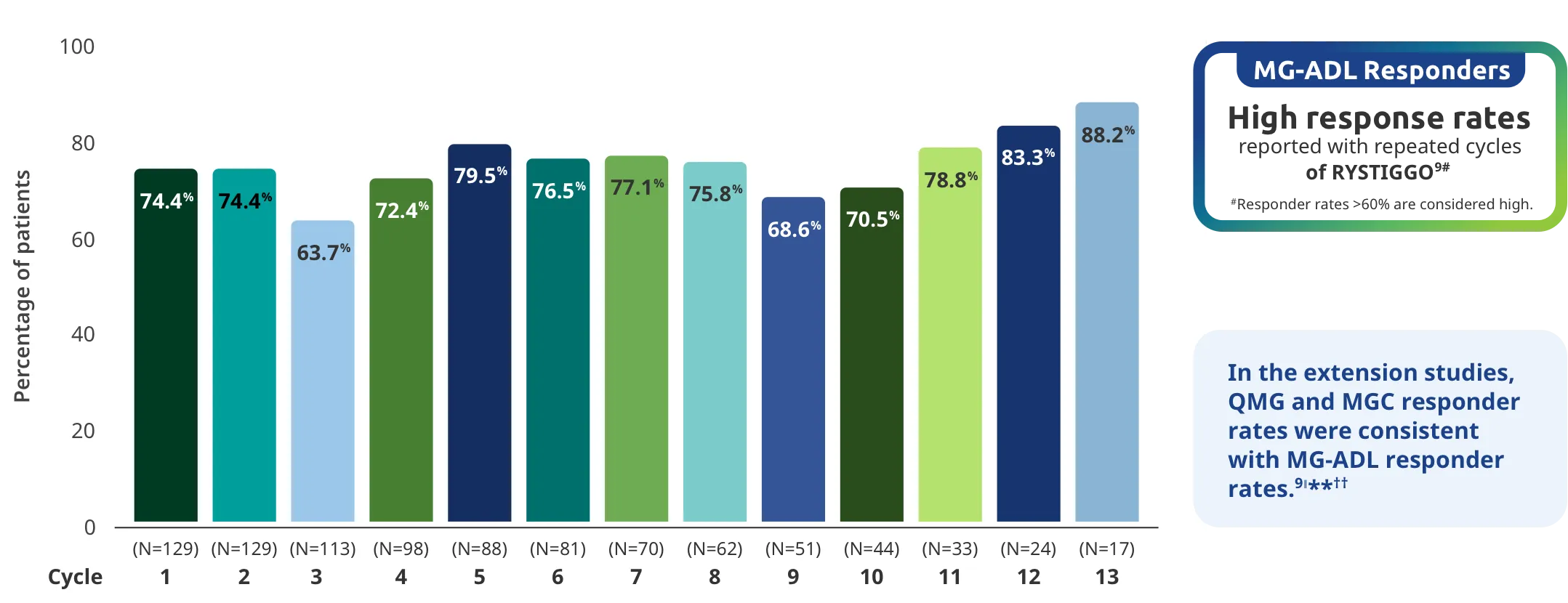
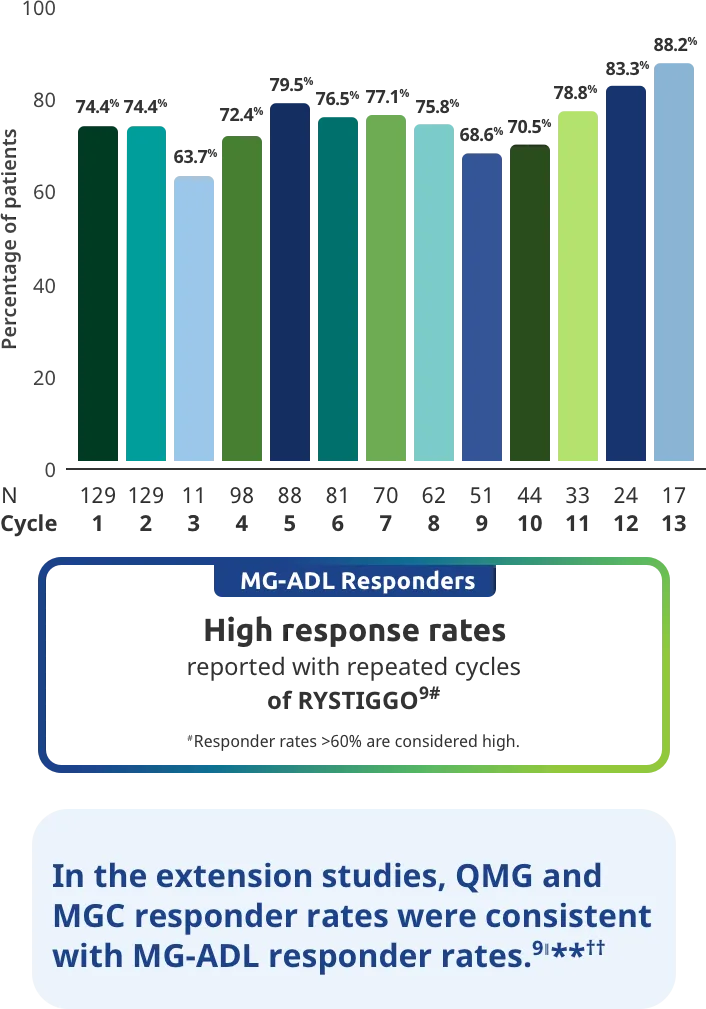
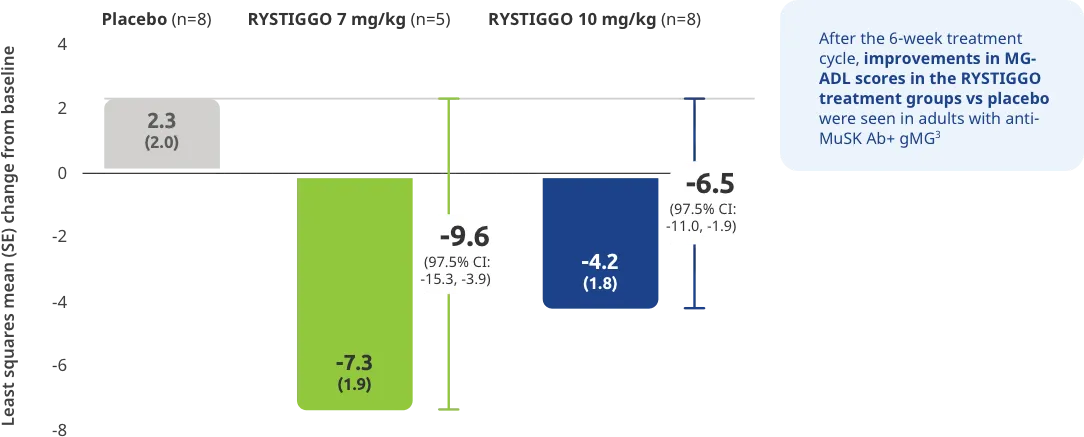
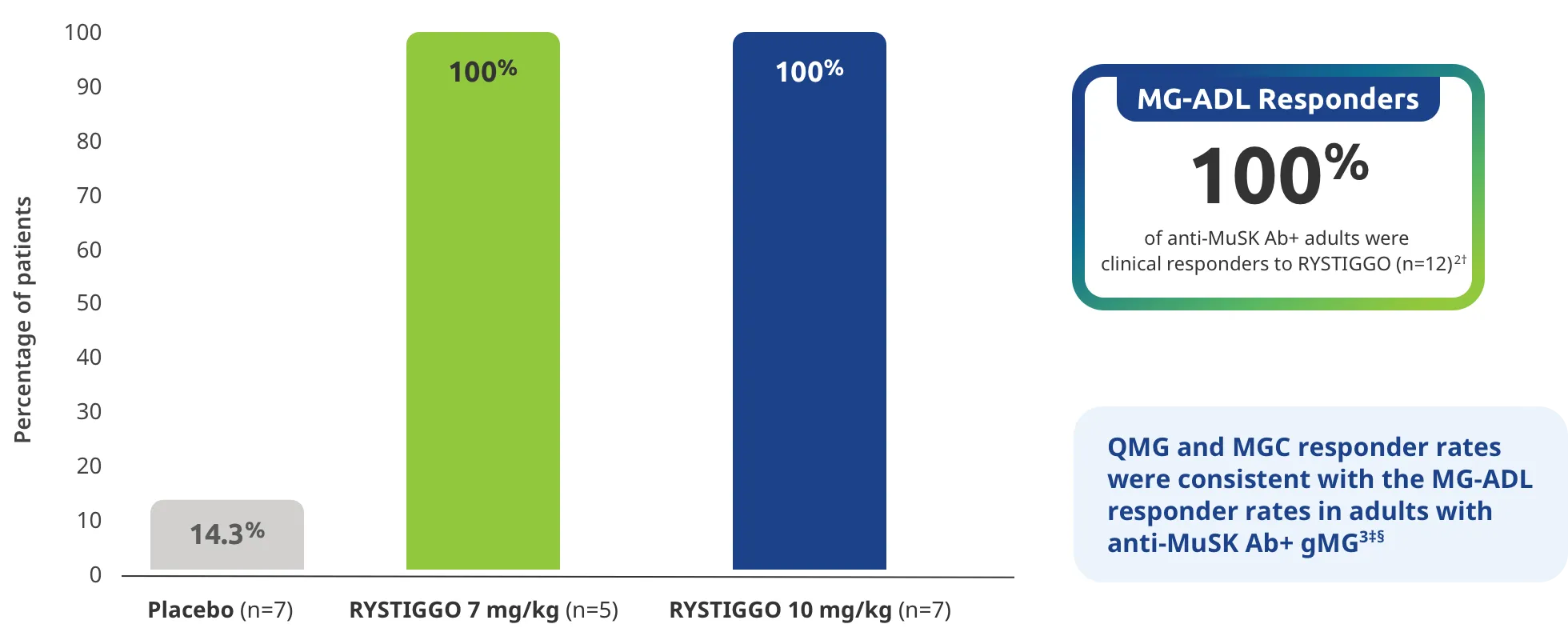
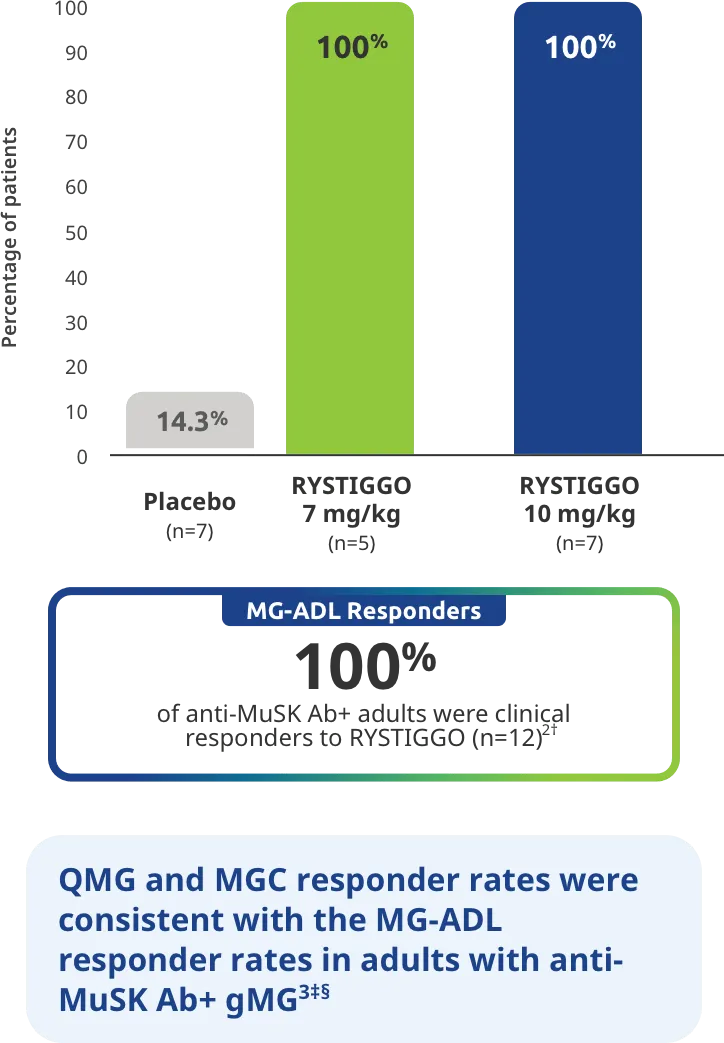
Adults taking RYSTIGGO experienced statistically significant and clinically meaningful improvements in activities of daily living at Week 61,2†
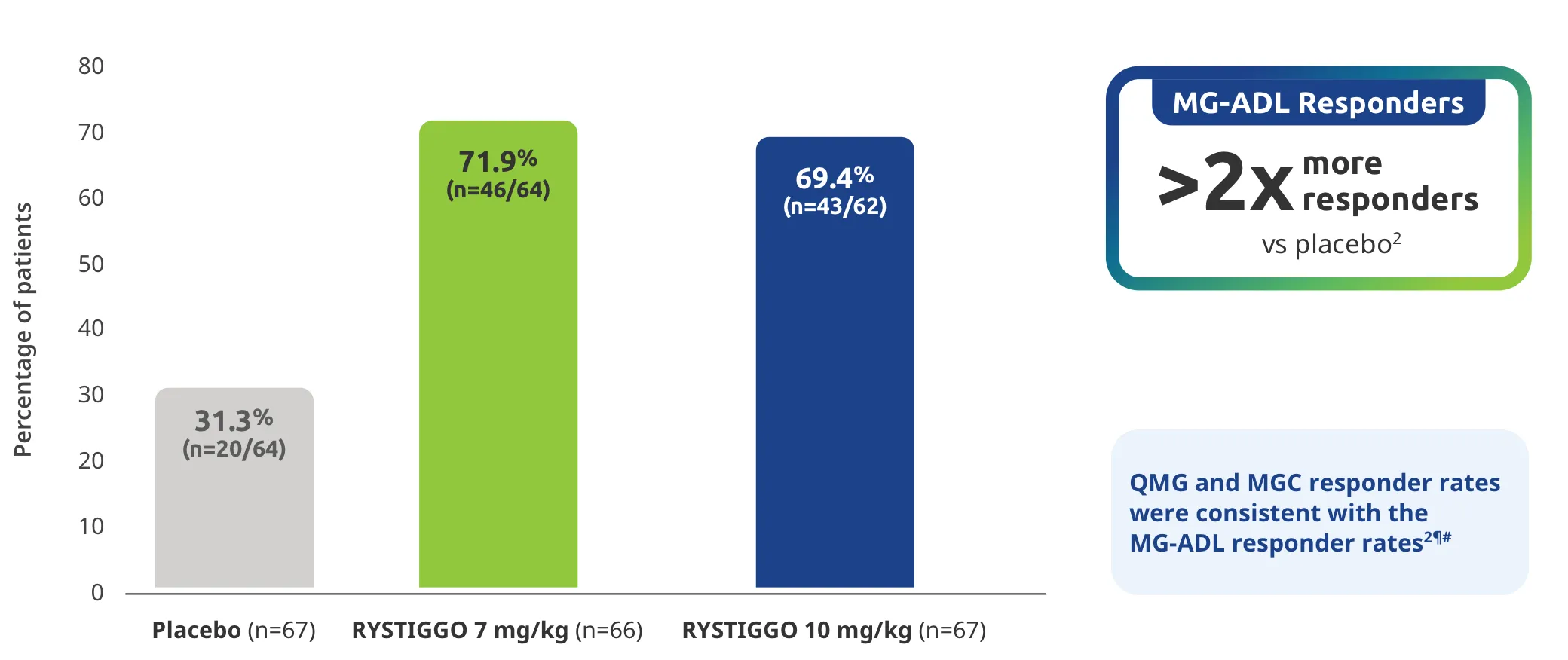
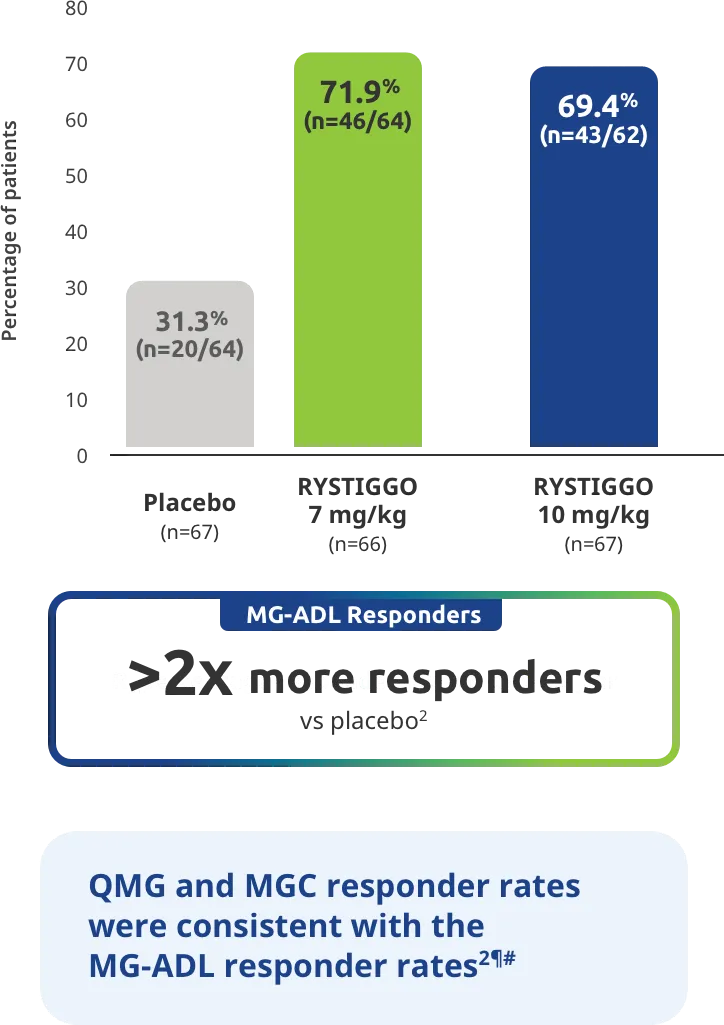
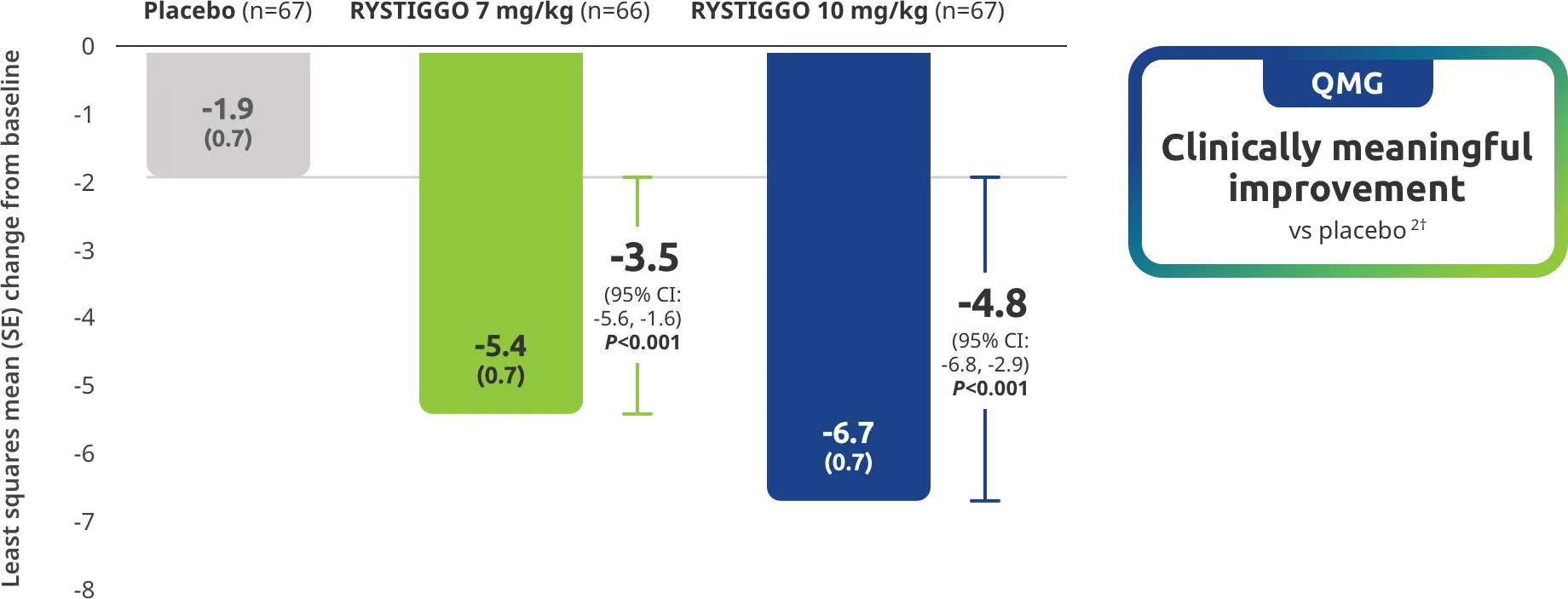
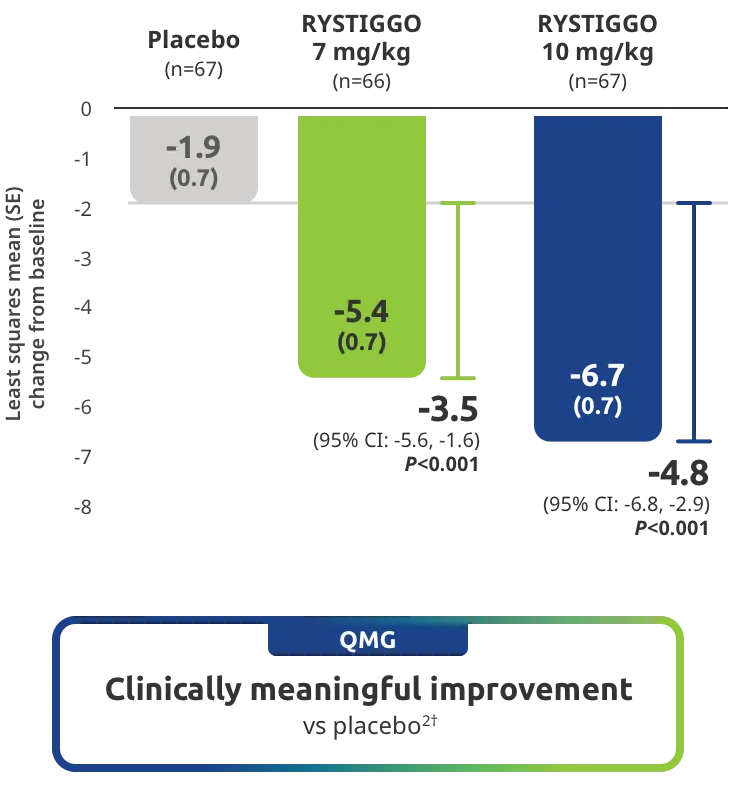
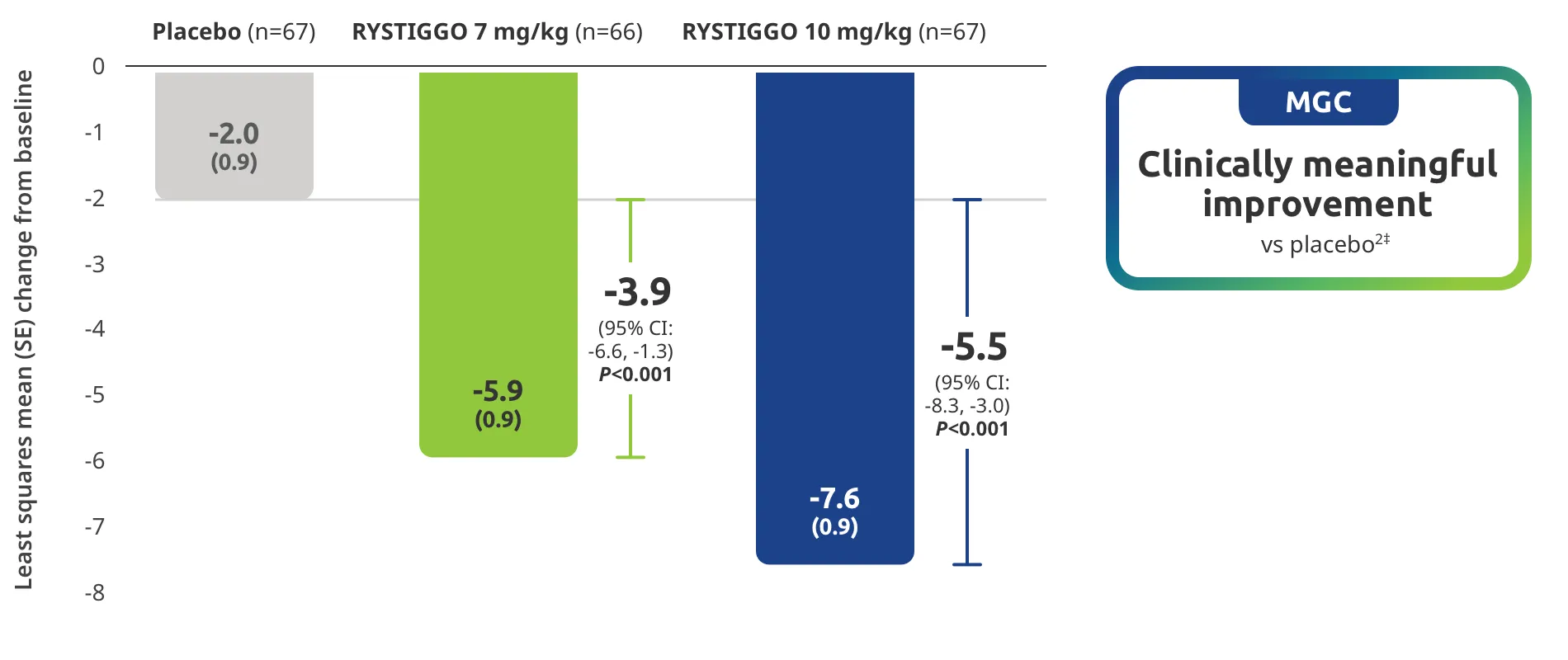
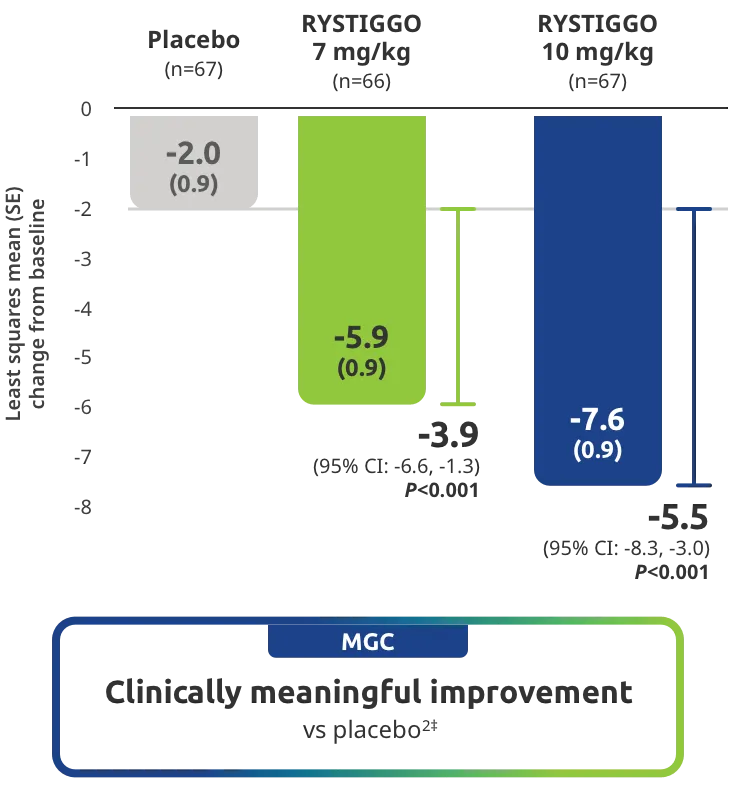
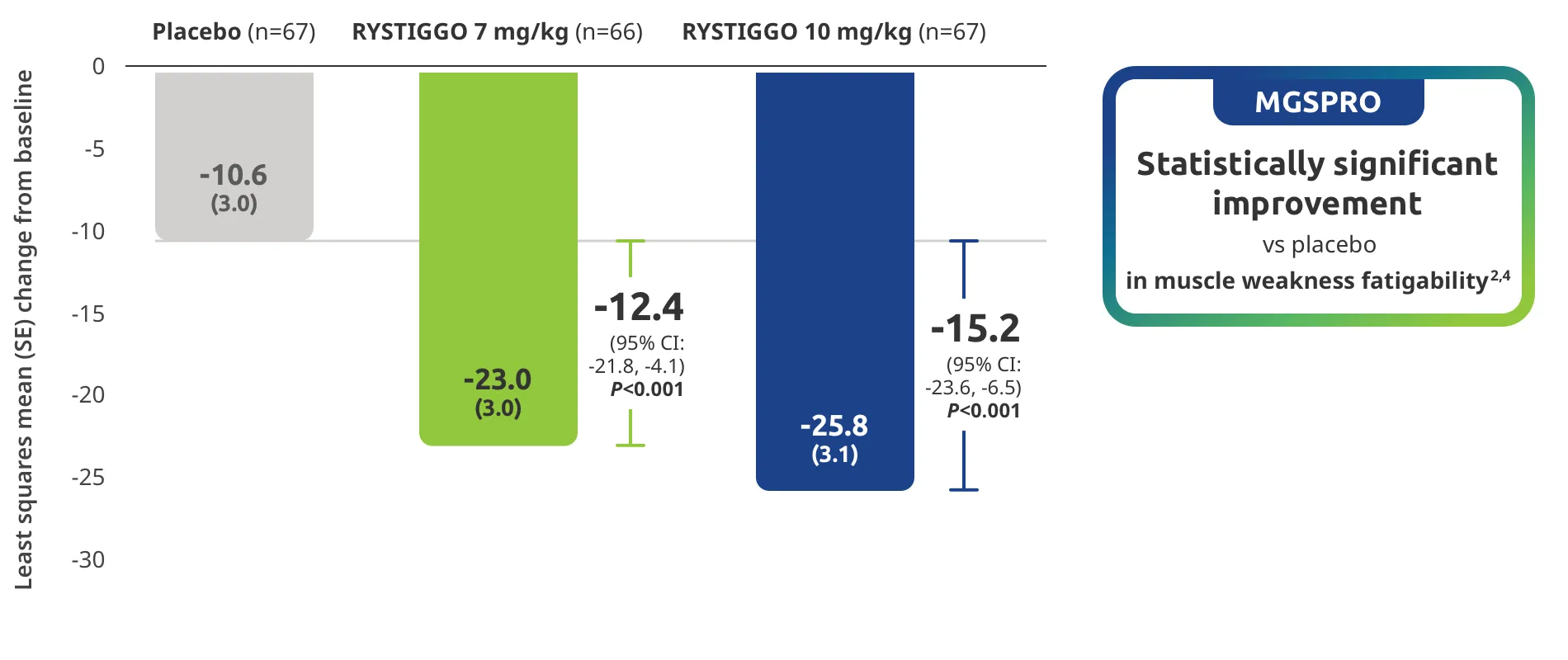
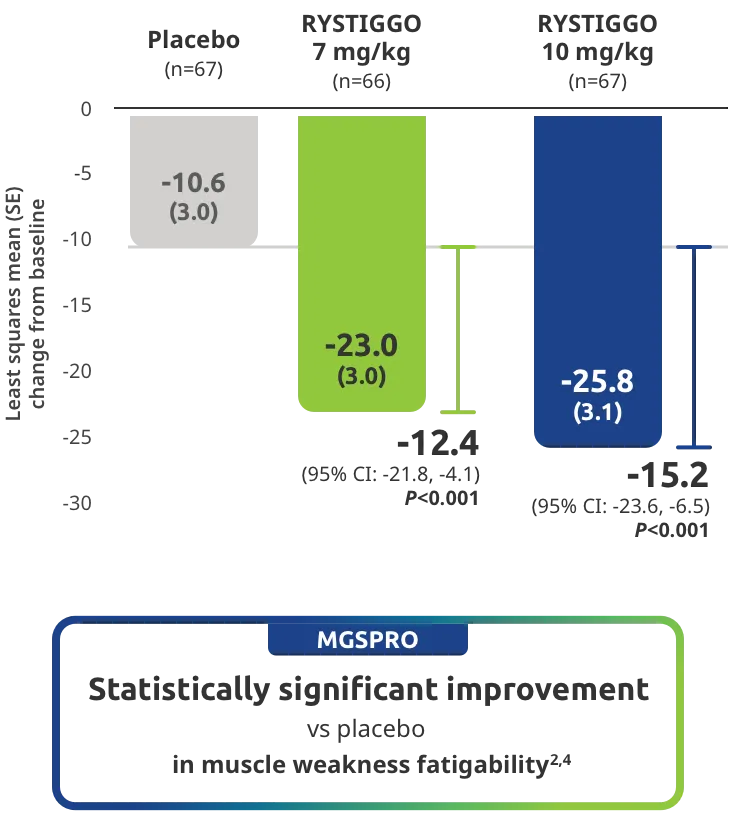
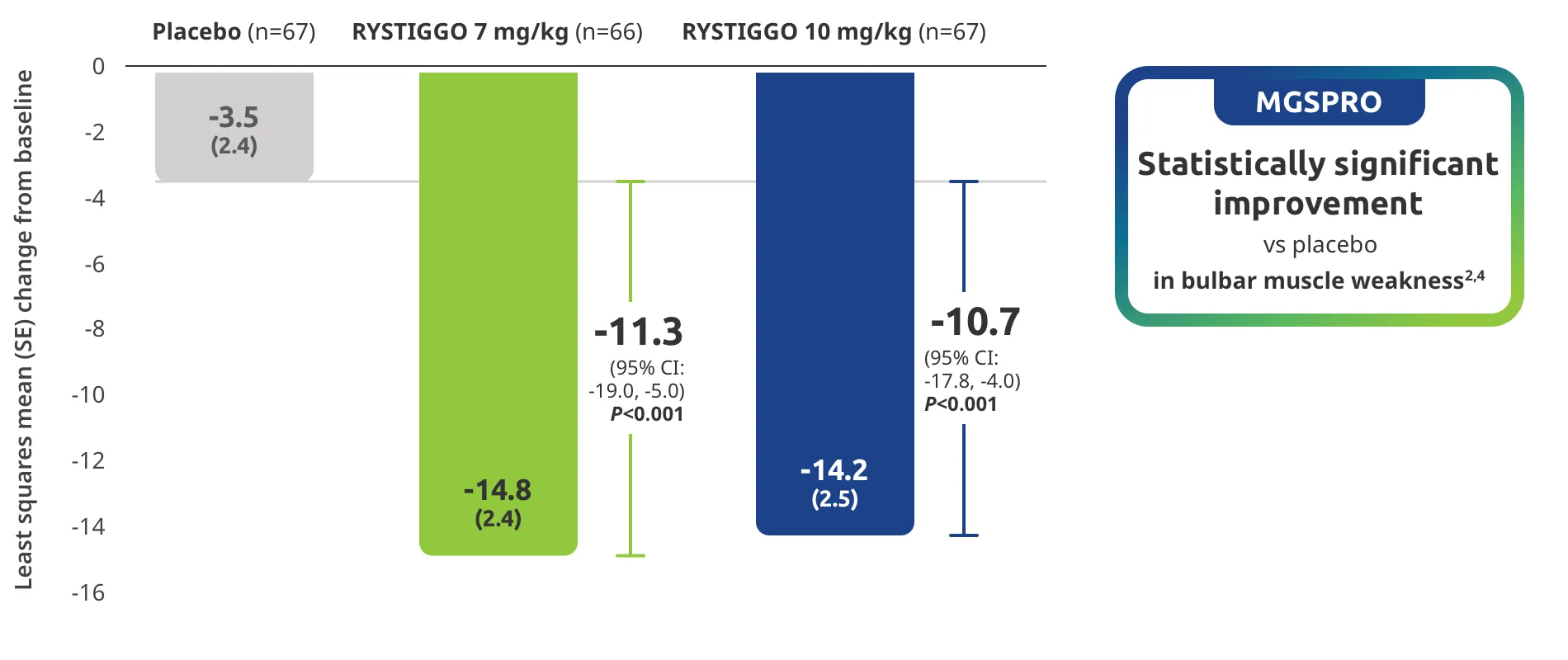
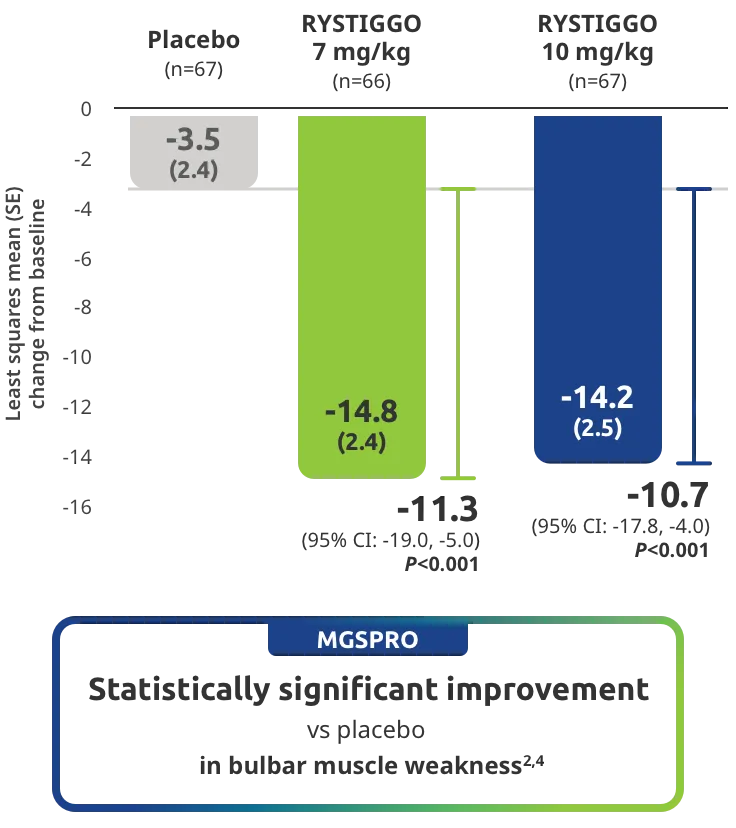
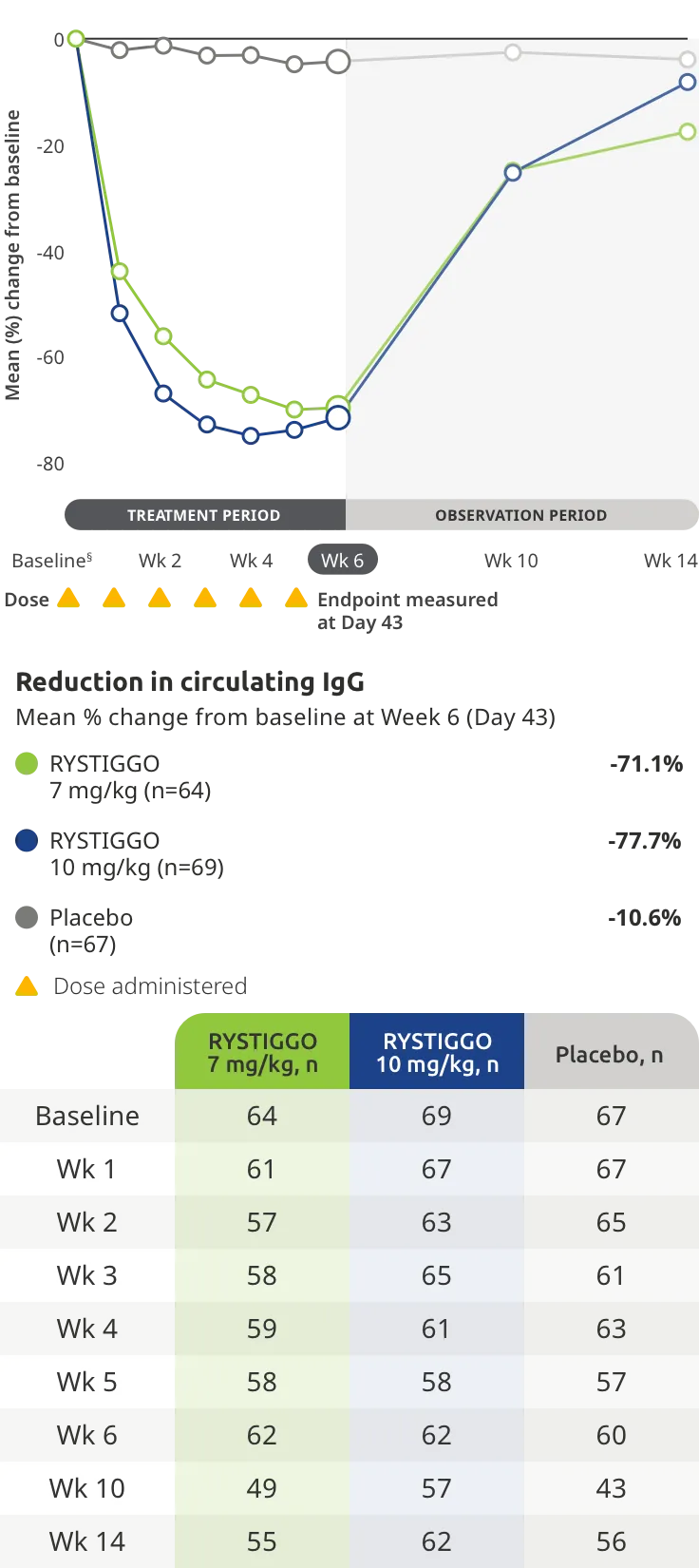
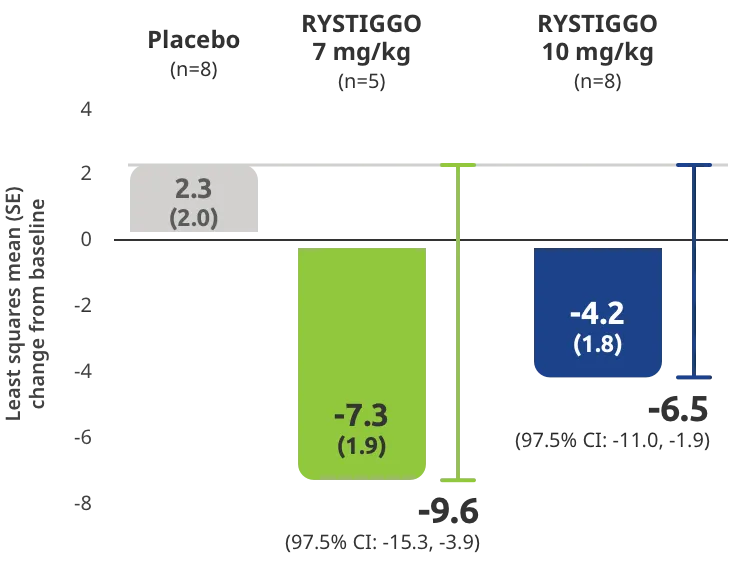
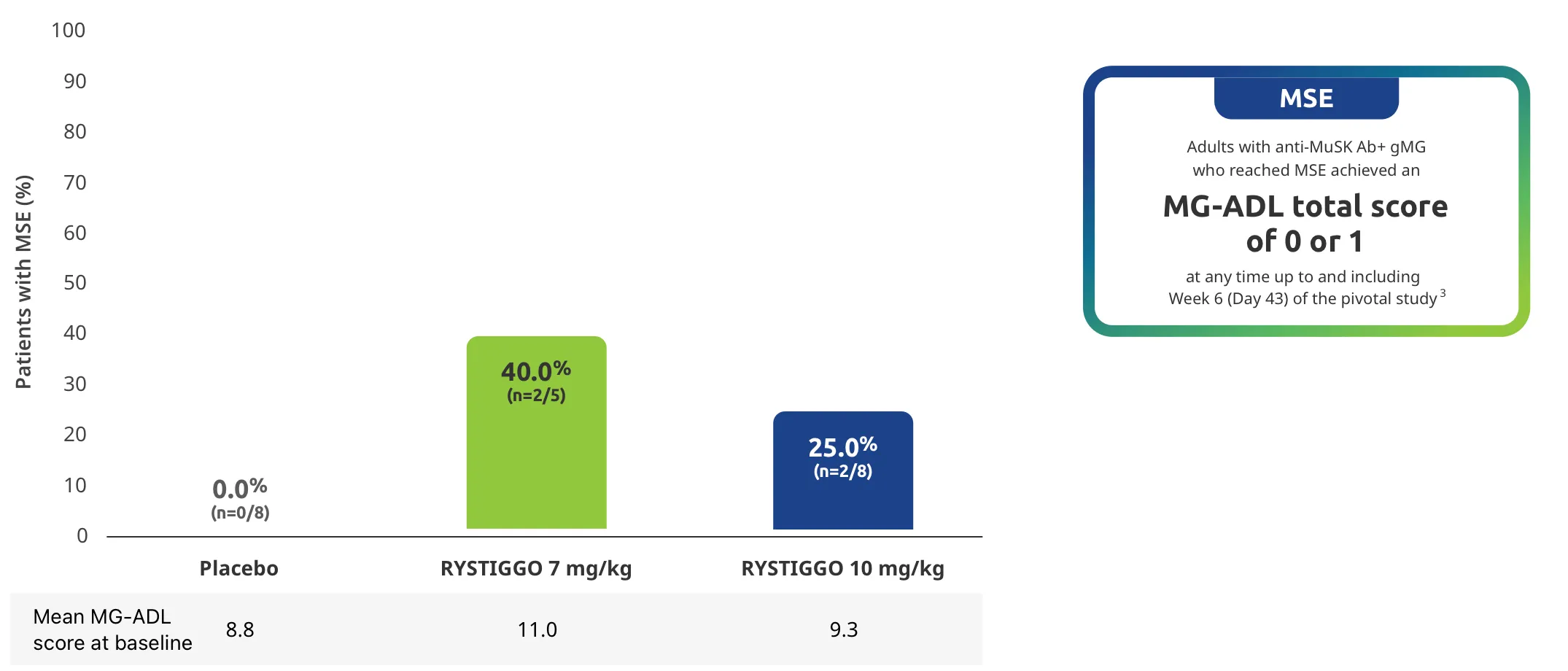
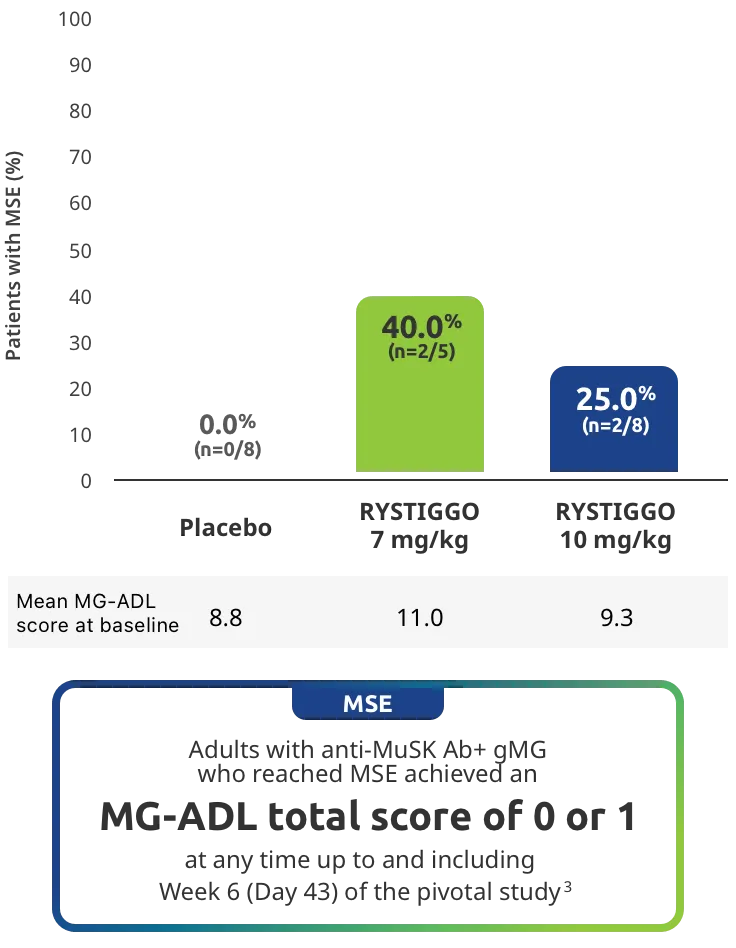
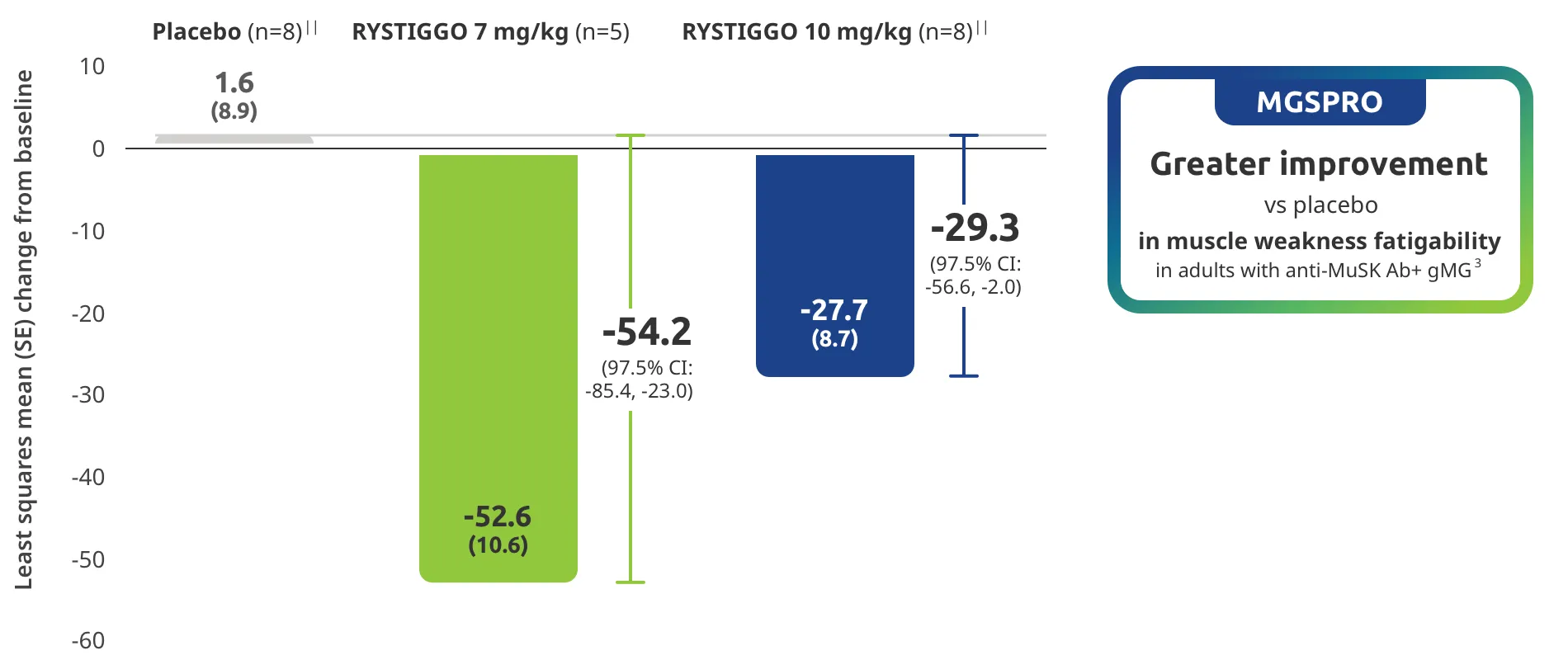
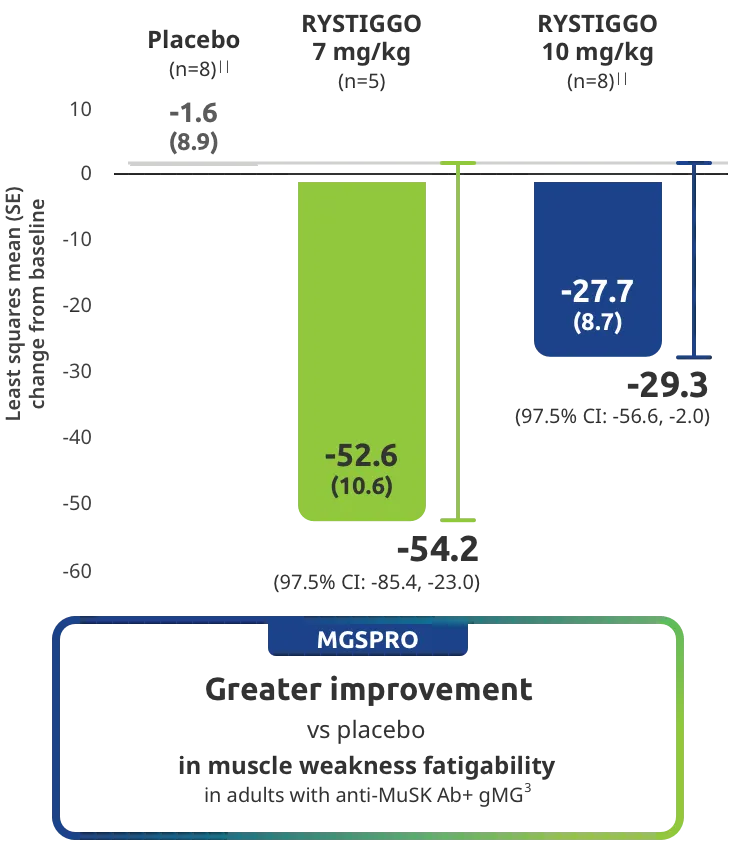
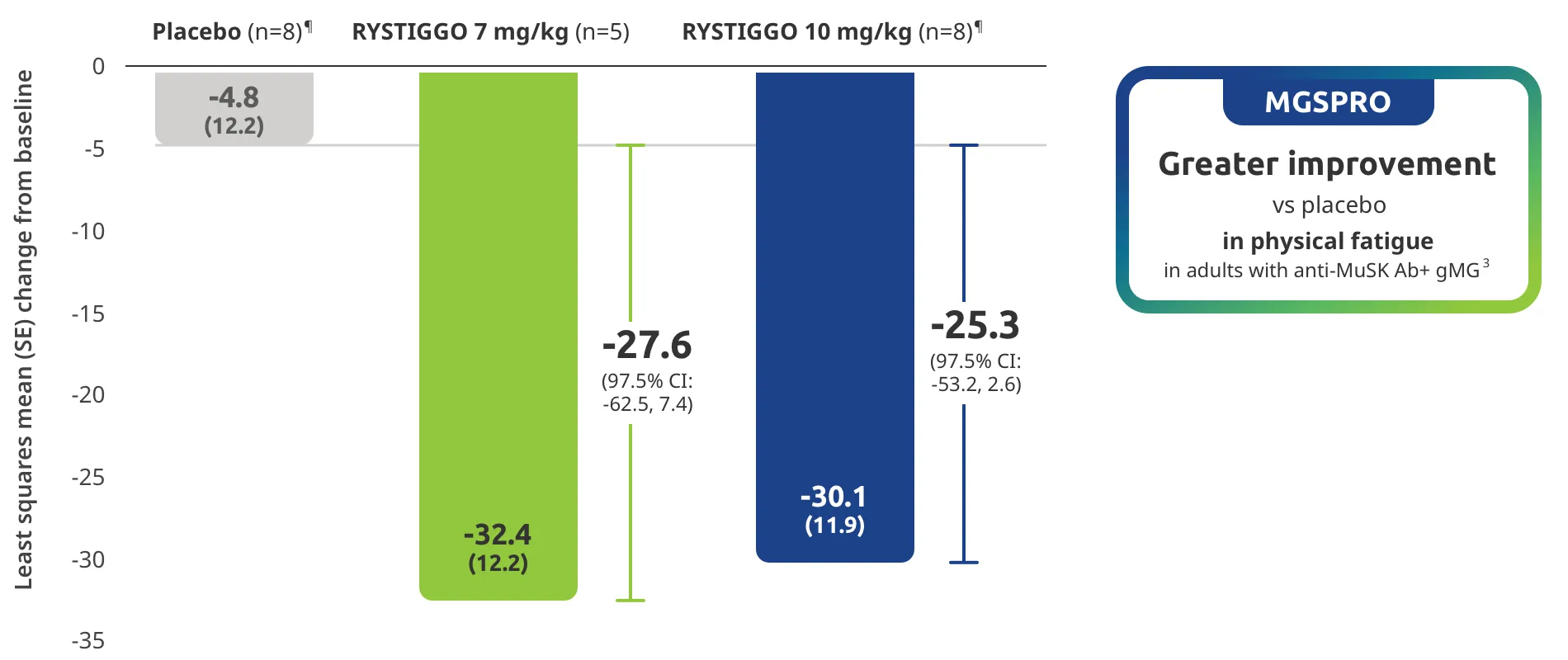
Change from baseline to Week 6 (Day 43) in MG-ADL total score in adults who are anti-AChR Ab+ or anti-MuSK Ab+1-3‡
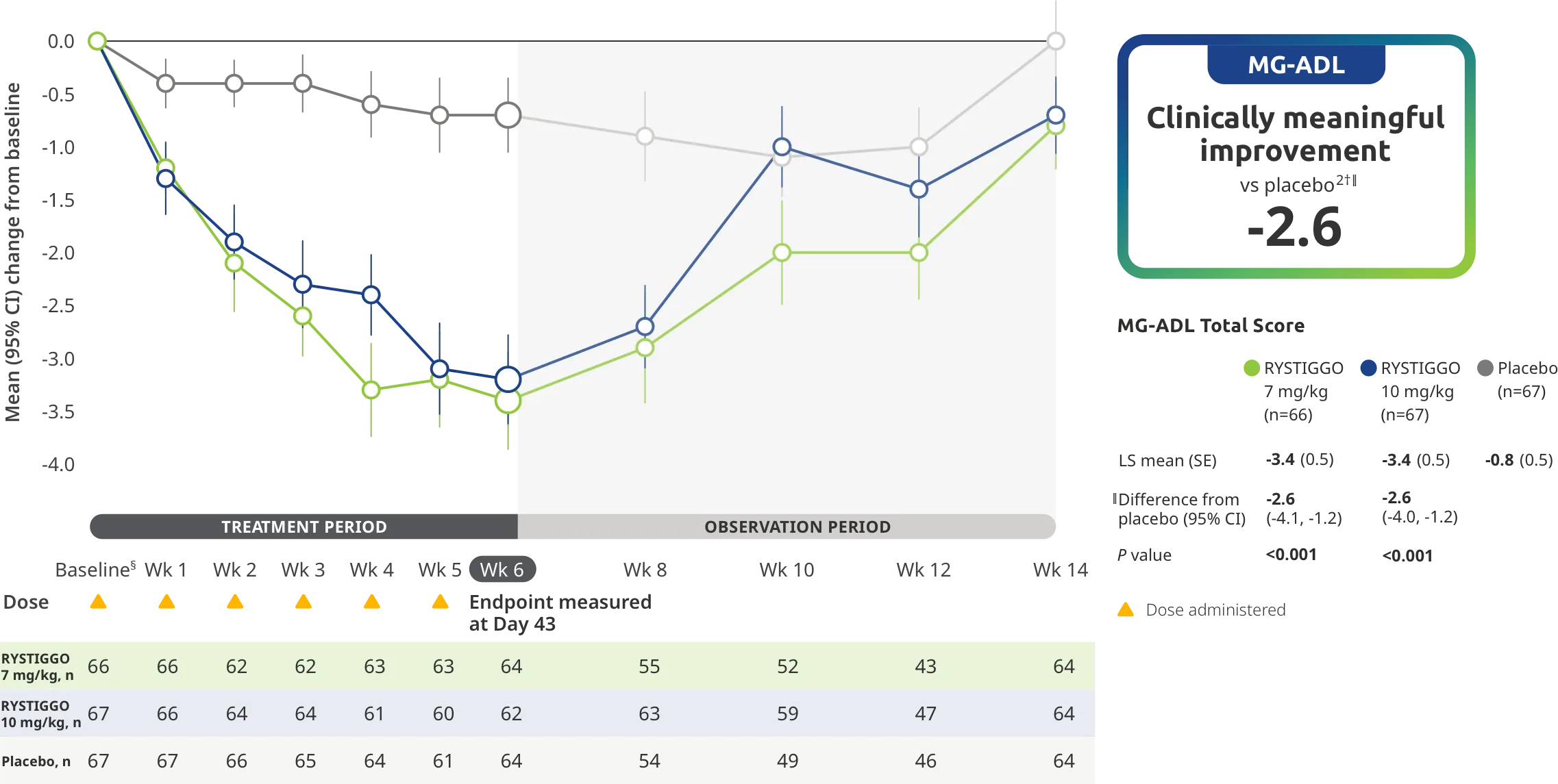
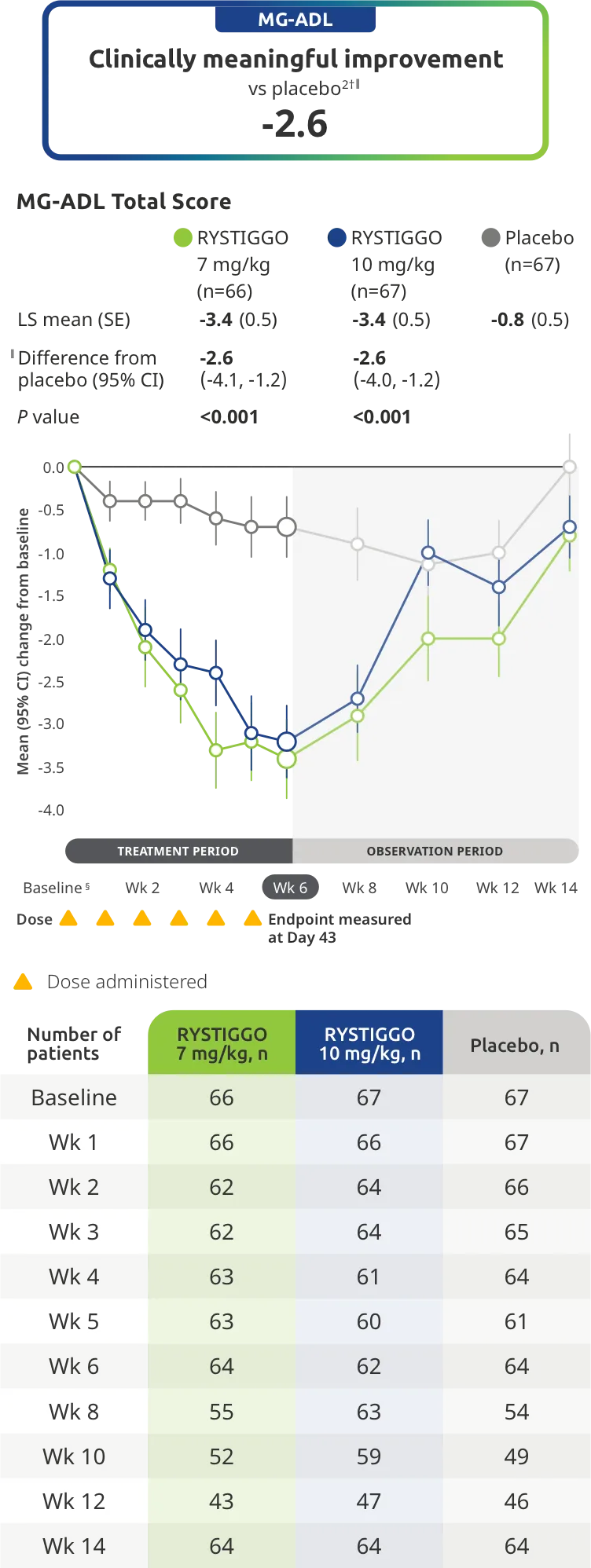
Clinically meaningful was established as a ≥2.0-point improvement in MG-ADL total score.2
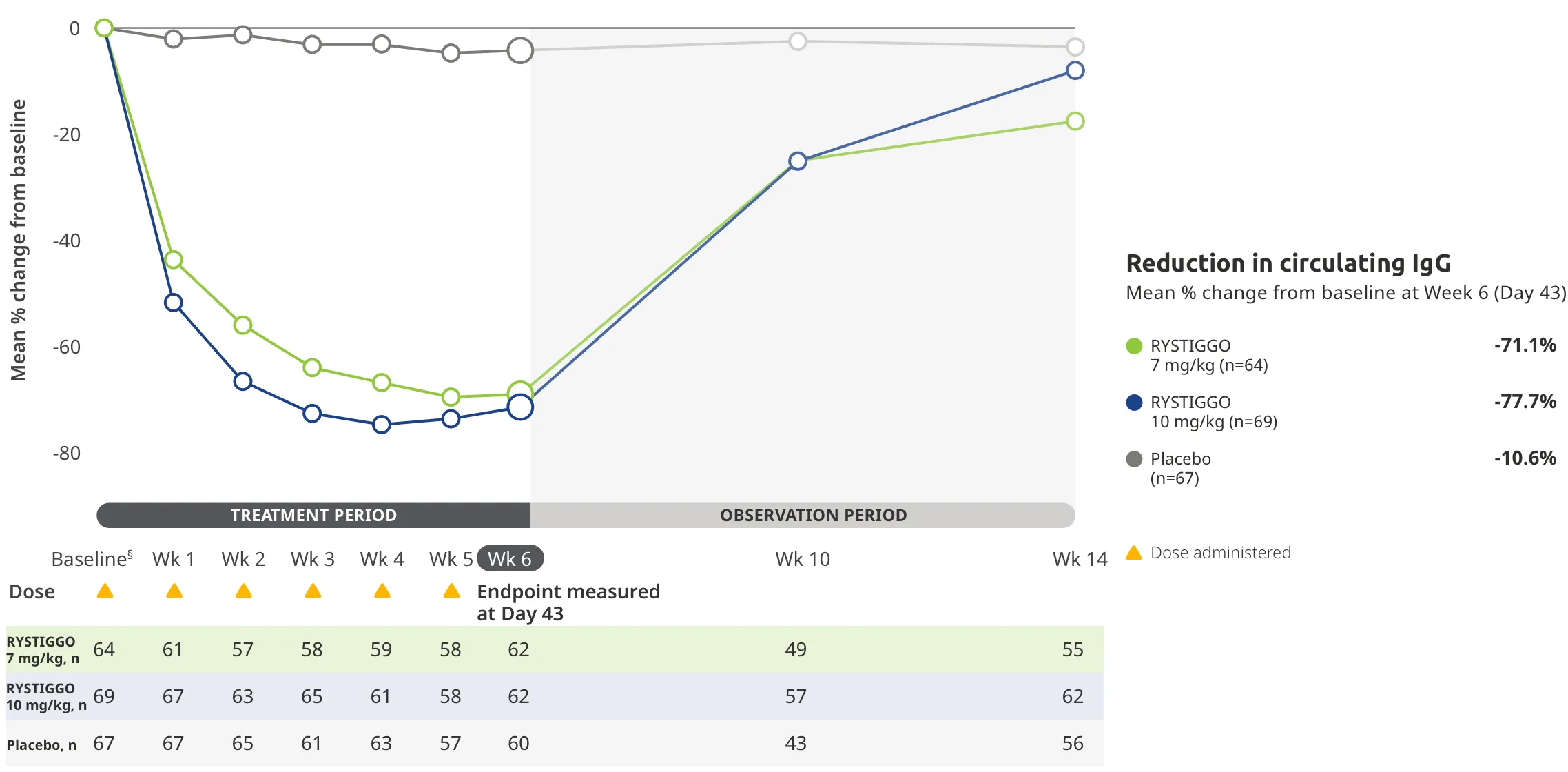
During the treatment period, RYSTIGGO or placebo were administered subcutaneously once a week for 6 weeks.1
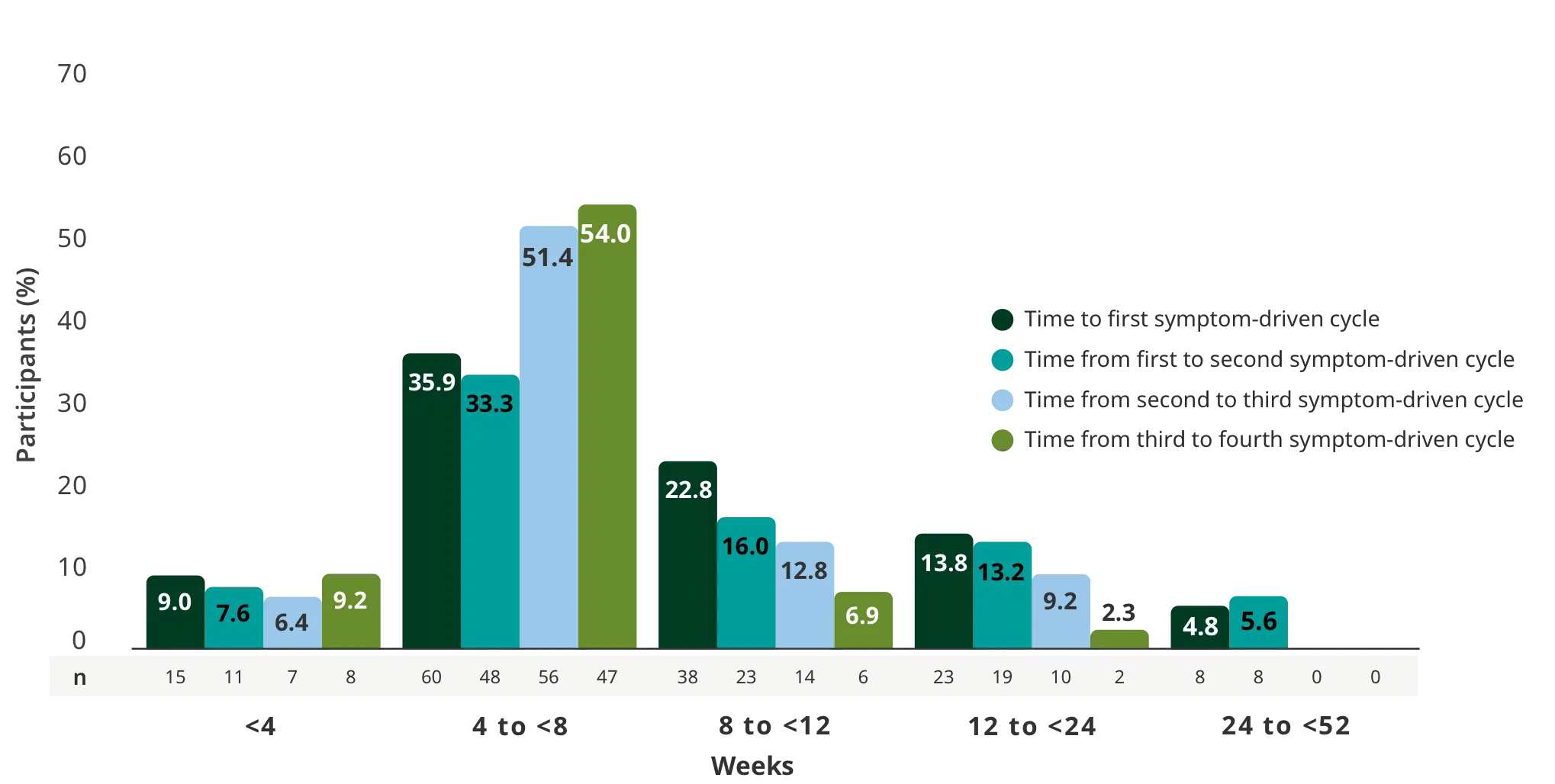
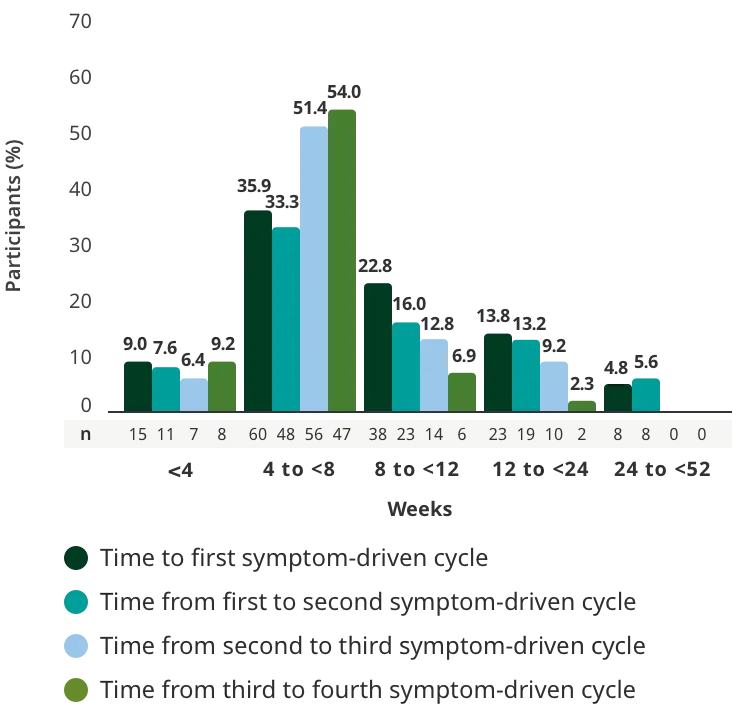
Treatment initiation on Day 1.2
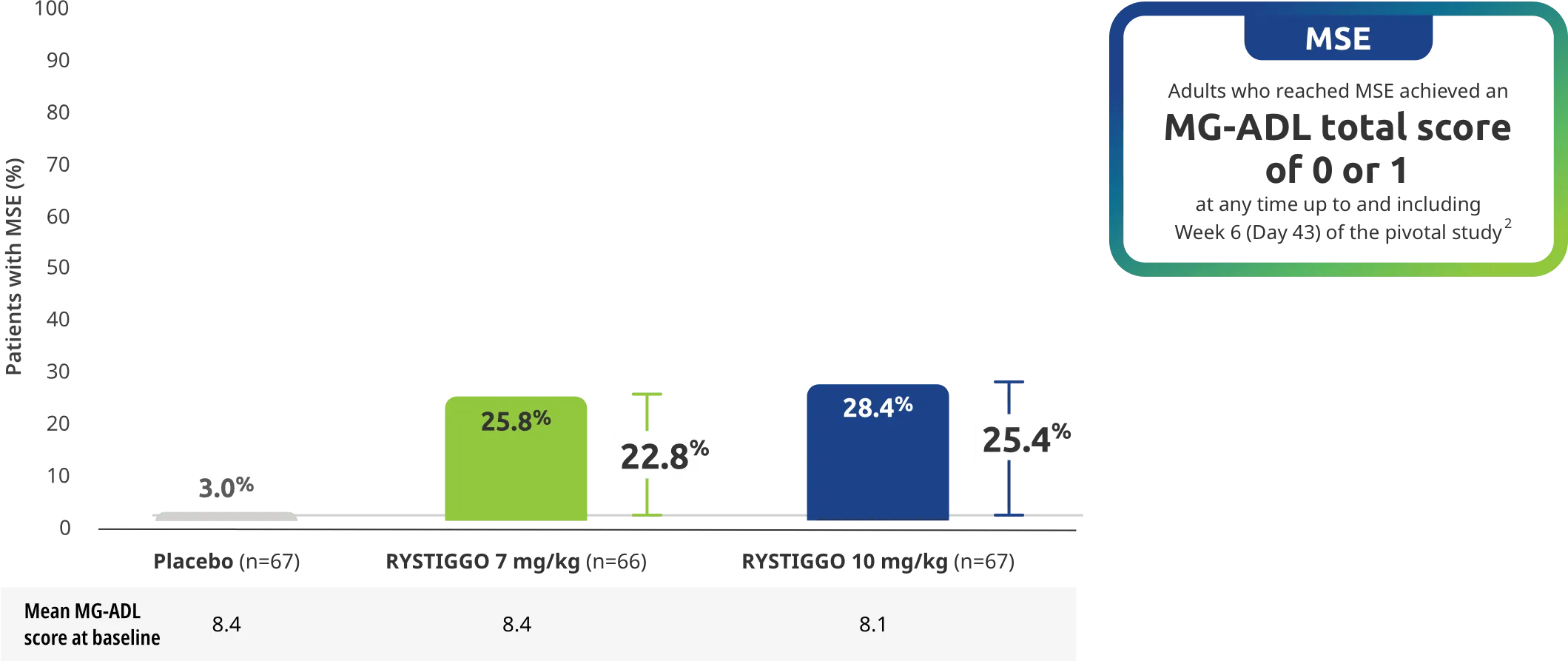
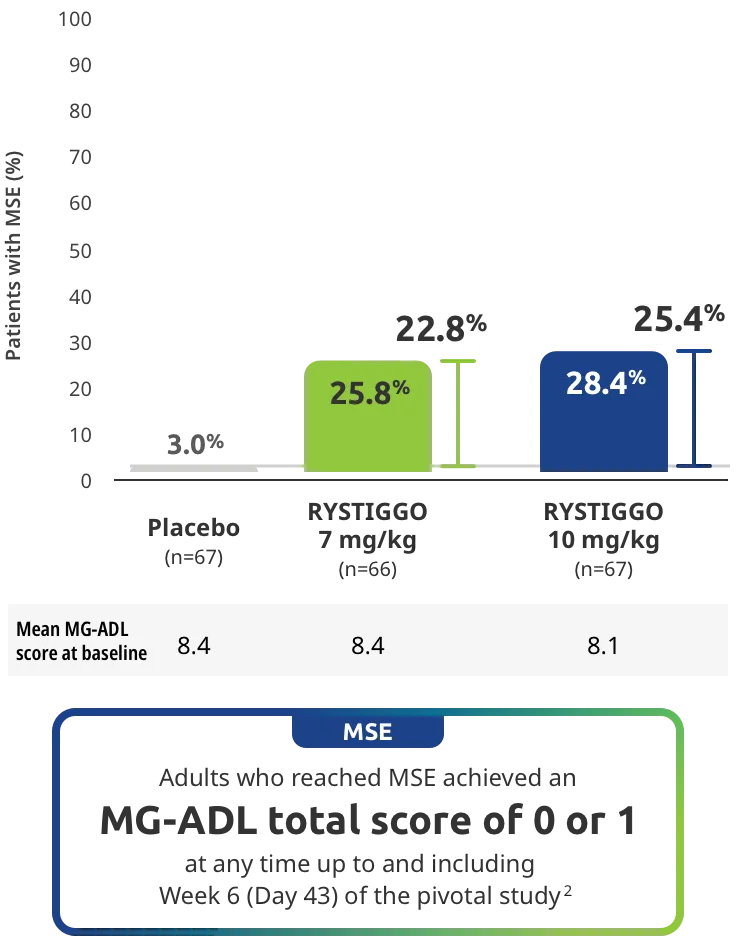
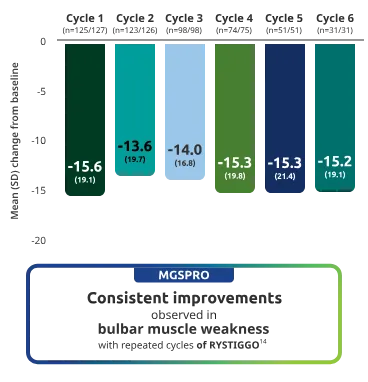
Adults taking RYSTIGGO achieved maximum efficacy at Week 6 (Day 43).1
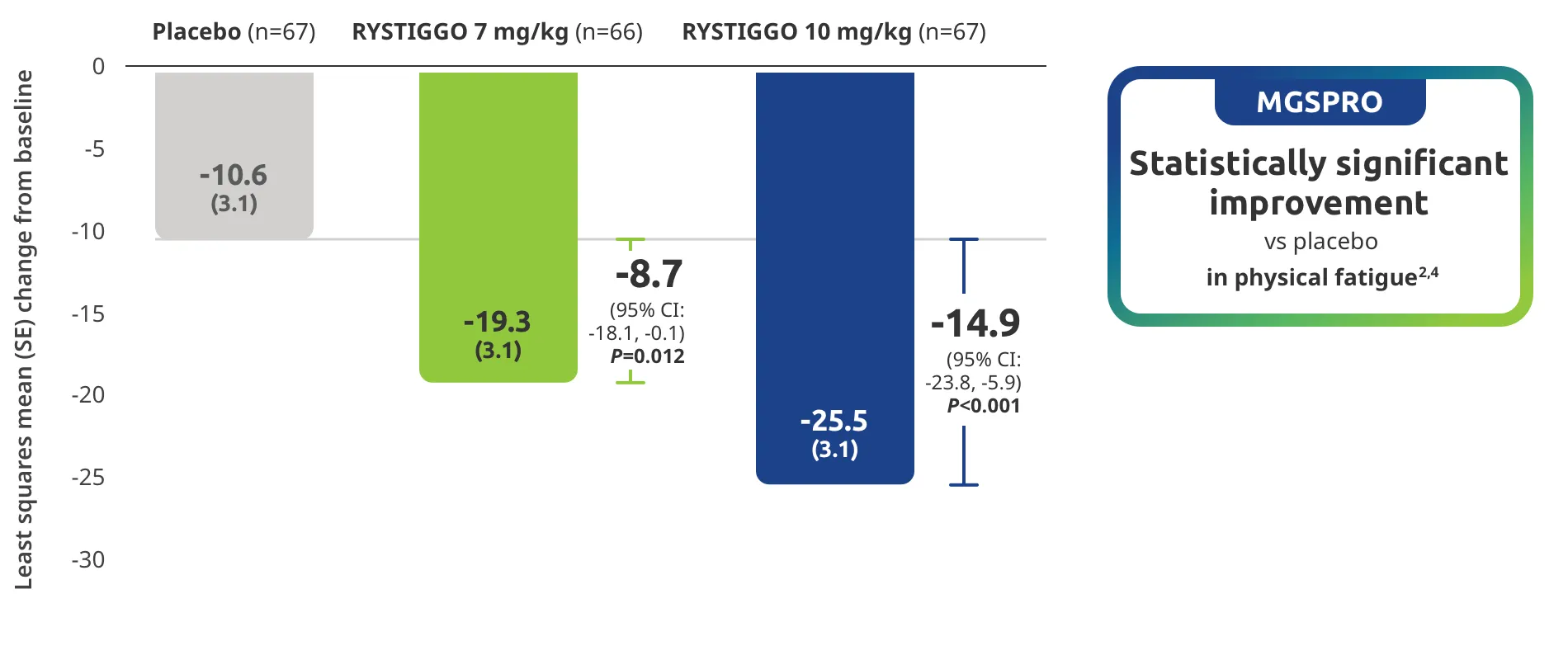
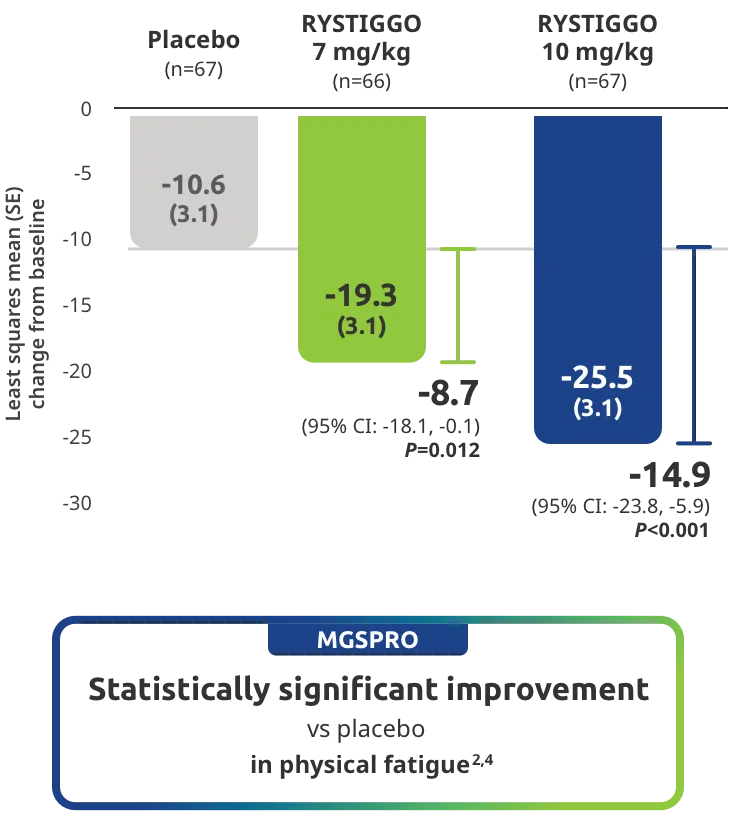
The efficacy of RYSTIGGO for the treatment of adults with anti-AChR Ab+ and anti-MuSK Ab+ gMG was established in an up to 18-week, multicenter, randomized, double-blind, placebo-controlled study. In the study, 200 patients were randomized 1:1:1 to receive weight-tiered doses of RYSTIGGO (n=133), either 7 mg/kg of RYSTIGGO (n=66) or 10 mg/kg of RYSTIGGO (n=67), or placebo (n=67). The study included a 4-week screening period and a 6-week treatment period followed by 8 weeks of observation.1
MG-ADL assesses the impact of gMG on daily functions of 8 symptoms on a scale of 0-3, with total scores ranging from 0-24. Higher scores are interpreted as greater impairments. An improvement of ≥2.0 points was established as clinically meaningful.1,2
Measures include4:
- Voice/speech problems
- Chewing
- Swallowing
- Breathing
- Brushing teeth and/or combing hair
- Rising from a chair
- Diplopia
- Eyelid droop
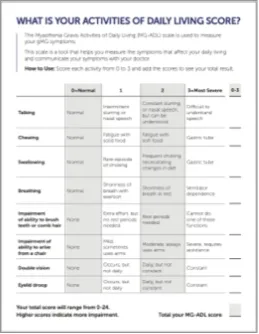
Have your patients monitor their progress with the MG-ADL guide